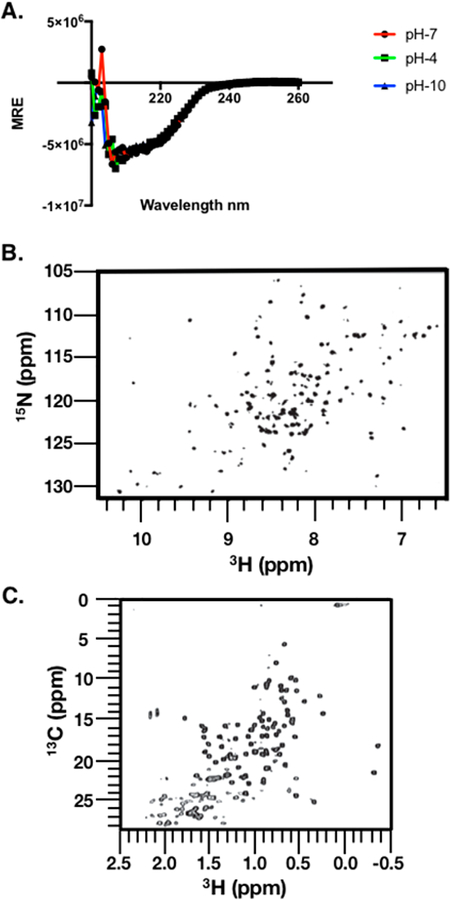
Figure 5.
Spectral mapping of the rhTIMP-2-6XHis structure. (A) Circular dichorism spectroscopy (CD spectra) of purified rhTIMP-2-6XHis. Purified, lyophilized rhTIMP-2-6XHis was reconstituted in buffer at pH 4 (acetate and 150 mM NaCl), pH 7 (Bis-Tris Propane and 150 mM NaF), and pH 10 (borate and 150 mM NaCl). Spectral scans were recorded at 37 °C between 180 and 260 nm. The CD signal of the mean residue ellipticity (MRE) is plotted vs wavelength.Scans with rhTIMP-2-6XHis at pH 7 (red), pH 4 (green), and pH 10 (blue) overlap almost completely, indicating no significant effect of pH on the overall secondary structure. (B) Amide fingerprint region from the 15NH, 1HN SOFAST-HMQC spectrum recorded at 15N natural isotope abundance. (C) Methyl fingerprint region from the 13C, 1H region recorded at 13C natural isotope abundance. All spectra were recorded at 900 MHz and 25 °C in 50 mM sodium chloride, 20 mM sodium deuteroacetate (pH 5.0), and 5% D2O.