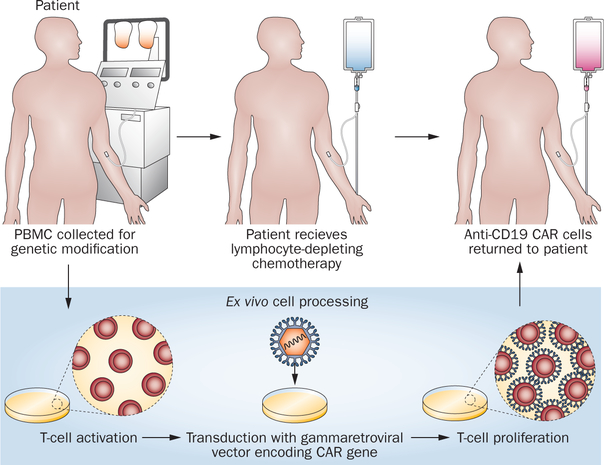
Figure 5 |.
A schematic of our current approach to anti-CD19 CAR T cell therapy is shown. The ex vivo cell processing takes 10 days. The lymphocyte-depleting chemotherapy regimen consists of fludarabine and cyclophosphamide. All patients receive 25 mg/m2 of fludarabine daily for 5 days. The cyclophosphamide dose depends on the patient’s platelet count. A cyclophosphamide dose of 60 mg/kg daily for 2 days is administered to patients with a blood platelet count of 100,000/μl or more. A cyclophosphamide dose of 30 mg/kg daily for 2 days is administered to patients with a blood platelet count between 75,000 and 99,000/μl. Patients with platelet counts less than 75,000/μL are not eligible for the clinical trial. Abbreviations: CAR, chimeric antigen receptor; PBMC, peripheral blood mononuclear cell.