Abstract
Objective
Natural-product dietary supplements (NDS), defined as non-mineral, non-vitamin, ingested natural product-derived substances, are the most frequently used CAM modality in the United States, with musculoskeletal disease being the most frequent reason for their use. Because NDS usage is frequently unreported and individuals with RA may be at higher risk for NDS-related side effects due the underlying nature of the disease and frequent use of complex pharmaceutical regimens, a scoping review of the literature was undertaken to examine population-based patterns of NDS use for RA self-management.
Methods
Using guidelines for scoping reviews, AMED, CINAHL, the Cochrane Library, Embase, Ovid/MEDLINE, and Web of Science databases were searched to identify references presenting primary data related to the prevalence or patterns of use of NDS in RA populations.
Results
Twenty-three studies, published between 1980–2015 and conducted in 11 countries, met inclusion criteria. The overall prevalence of NDS use in RA was 47% worldwide and did not differ by geographic region. On average, 47% of subjects found NDS to be effective and 13% reported adverse side effects, with only 30% informing their physicians about use of NDS, which were used concomitantly with RA pharmaceuticals in a majority of cases. Marine oils, glucosamine, vinegar and chondroitin were among the most commonly reported NDS worldwide.
Conclusion
Given the apparent communication gap between patients and providers regarding NDS use and higher potential risks associated with this usage in RA, ongoing surveillance of population-based practices may help facilitate RA management and direct future NDS research.
INTRODUCTION
Natural-product dietary supplements (NDS), defined as non-mineral, non-vitamin, systemically ingested natural-product derived substances, are the most frequently used form of complementary and alternative medicine (CAM) in the United States (US), with approximately 18% of US adults reporting NDS use (1). Because treatment of musculoskeletal conditions is the most common reason given for CAM use by US adults (2), it is not surprising that a much higher prevalence of dietary supplement use is reported by adults with arthritis (up to 62.5%) (3). Despite a paucity of evidence supporting efficacy, NDS use has continued to rise in the US, where herbal supplements sales have increased annually at a rate of 6–8% since 2003 (4). Given the well described potential for adverse interactions between concurrent NDS and prescribed medications (5), this apparent increase in NDS use may lead to population-specific safety hazards that may, in addition, go unrecognized since up to 76% of patients do not discuss their use of dietary supplements with their physicians (6).
Individuals with rheumatoid arthritis (RA), a particularly aggressive inflammatory polyarthritis associated with systemic complications and significant morbidity and disability, comprise one such population that may be at increased risk for NDS/drug interactions. Conventional RA therapy includes early and ongoing treatment with disease-modifying anti-rheumatic drugs (DMARDs) and biologic therapies with established efficacy in reducing disease activity that are also associated with well documented risks, such as life-threatening infections, malignancies and major-organ dysfunction, (7, 8) and a high annualized cost ($26,000 USD) (9). These potential risks of conventional therapy, when coupled with the perceived safety of NDS, could increase the prevalence of NDS use for RA symptom management, a practice that could place RA patients at a higher risk of adverse side effects if coupled with intensive pharmacological management.
Given a general paucity of information regarding NDS efficacy or safety in RA, as well as the communication gap between patients and their health care providers regarding NDS use, a scoping review of the literature was undertaken to identify RA population-based studies documenting NDS use for RA self-management, including the prevalence and identity of specific NDS products used by RA patients in various countries, including the United States.
METHODS
Design of Systematic Literature Search
Because NDS use in RA populations is neither well defined in terms of types of products used, including standardized definitions of which products are considered to be NDS, nor comprehensive in terms of defining RA-specific risks and benefits of those NDS that are commonly used, we elected to cast a wide net to capture as much reputably published information documenting NDS usage by RA populations as possible A systematic scoping methodology was therefore adopted for this review, based on Daudt et al.’s definition that “[s]coping studies aim to map the literature on a particular topic or research area and provide an opportunity to identify key concepts; gaps in the research; and types and sources of evidence to inform practice, policymaking, and research.(10)
A literature review was conducted using recommendations for scoping studies as outlined by Arksey (11) and further refined by Levac (12) which, briefly, consist of five steps: 1) identifying the research question (prevalence and types of NDS used for RA self-management); 2) identifying relevant studies; 3) selecting studies; 4) charting the data; and 5) collating, summarizing, and reporting the results, using methodology specified in the PRISMA statement for reporting systematic reviews and meta-analyses (13). To identify relevant studies, a systematic literature search, completed on July 10, 2017 (CLH), was designed using both controlled vocabulary terms (e.g., MeSH, EmTree) and keywords to search the following six databases: AMED (Allied and Complementary Medicine) 1985–2017; EBSCO/CINAHL (1937–2017); Wiley/Cochrane Library (1898 −2017); Elsevier/Embase (1947–2017); Ovid/MEDLINE (1946–2017); and Thomson-Reuters/Web of Science (1898–2017). The complete Ovid/MEDLINE search strategy, analogous to the other database searches, is available as Supplemental Figure 1. Additional citations listed within studies ultimately selected for review or from prior reviews were also screened. No publication date, study type, or language limits were applied. An abstract representing work-in-progress by co-authors of this review was excluded (14).
Methodology for Assessing Inclusion
Titles and abstracts of the retrieved references thus identified were reviewed for inclusion by two independent reviewers (JCD, MBS), with cases of disagreement resolved by consensus among all authors. A similar process was used to screen the full text of selected studies, with final decisions made by JLF. Studies were selected for inclusion using the following inclusion criteria: 1) at least 50% of study participants in a data set were individuals with a diagnosis of rheumatoid arthritis; 2) the study reported on the extent or patterns of use of natural product/dietary supplements, defined as non-mineral, non-vitamin, systemically ingested biologic compounds. Studies had to include values for either overall prevalence of NDS use or prevalence of use for specific products or both. Specifically excluded were studies whose participants had non-specified types of arthritis (or other diseases) or where RA constituted ≤50% of a defined study population or was unknown. Also excluded from the analysis were articles that did not give a definition of NDS similar to that used here and/or identify examples of NDS consistent with this definition; studies presenting NDS data unrelated to usage patterns (e.g. mechanism of action, efficacy or prescribing patterns); and review or opinion/commentary articles lacking primary data. Lastly, because patients frequently do not report NDS use to their providers, studies based solely on chart reviews or databases lacking specific queries related to NDS use were not included.
Data Extraction
All included studies were read and data were extracted that pertained to the RA patient subgroups and use of NDS. Data extracted from the n = 23 articles thus selected included the year of publication, country of the study, methodology of data collection, subject number and demographics, prevalence of NDS users and identity of specific supplements used, when available. Other variables recorded, when available, included prevalence of patients who perceived NDS to be effective, rate and description of reported adverse reactions and whether patients informed their healthcare providers of NDS use.
Data Synthesis and Analysis
Available data regarding overall prevalence of NDS use (n = 19 of the 23 studies) for products most closely matching our definition (non-mineral, non-vitamin, systemically ingested natural product derived substances) were extracted and compared across studies, a task made difficult by the immense heterogeneity in the definition for NDS amongst studies, the various methods used to define dietary supplement subgroupings (e.g. inclusion of fish oil or vitamins as herbals), and variances in the scope of data collected. Studies that reported data on non-RA patients and/or on CAM modalities in addition to NDS were included in this review if: 1) data on RA patients and/or NDS use were reported separately, or 2) the majority (> 50%) of the study population had RA and/or NDS accounted for the majority of reported CAM usage. When prevalence of use data for “nutritional supplements”, “herbs taken orally” or “traditional medicine” were reported separately, the subgroup of products most similar to our definition was determined by examining examples of substances in each category, using a single category to calculate the average prevalence of NDS use across all studies. Vitamin and mineral supplement usage data were excluded from this analysis when reported separately from NDS, although many of the studies included use some vitamins in their calculation of NDS prevalence. For those studies reporting data on the prevalence use of specific NDS products (n = 16 of the 23 studies), the top 5 most frequently used NDS from each study were recorded and the number of studies in which these substances were reported was determined. To calculate average prevalence of specific NDS product usage, only those studies where a prevalence of use for that specific supplement was reported were included in the calculation (including studies with prevalence = 0%). We excluded any study that did not report data on that supplement from the calculation entirely since it could not be ascertained whether the studies were designed to capture data on every possible supplement. Some types of food products used medicinally that were analogous to botanical extracts, such as gin-soaked raisins or vinegar, were included, while brand-name and combination supplements were excluded from this analysis. Data were analyzed using Prism software (Graphpad, San Diego), with results reported as mean, median or range, as indicated.
RESULTS
Identification of Relevant Studies
Searches of the six databases identified 4857 articles (Figure 1). Citation analysis of the most relevant studies revealed an additional 23 articles. Of the 3597 articles that remained after duplicates were removed, all but n = 72 were excluded due to irrelevance to the topic (Figure 1). Twenty three of these 72 articles met the full set of inclusion criteria after full text review, (15–37) including six studies available only as conference abstracts (17, 22, 30, 34–36).
Figure 1.
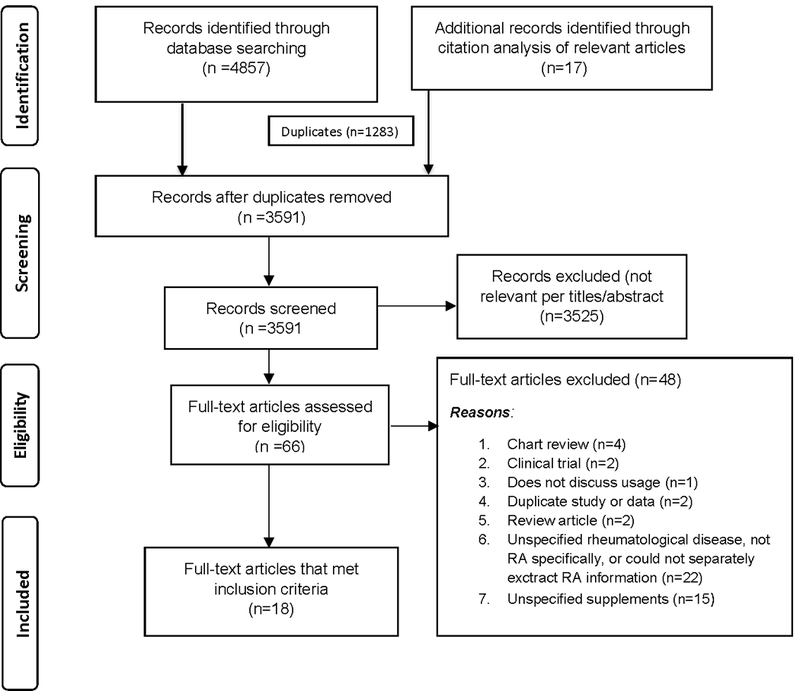
PRISMA flow diagram illustrating the process for literature search and extraction of publications (n = 23) meeting criteria for inclusion (13).
Study Characteristics (n = 23 studies; Table 1)
Table 1.
Summary of NDS use in RA (n = 23 studies)
| Country (State/Region) | Year published | n (RA subgroup) | Study Population | Prevalence of NDS Use (%) | Most frequent NDS reported | Effectiveness perceived (%) | Adverse events reported (%) | Informed health care providers of usage | Using concurrent prescribed treatment (%) | Author |
|---|---|---|---|---|---|---|---|---|---|---|
| THE AMERICAS | ||||||||||
| USA (CA) | 1980 | 384 | Community patients under surveillance by arthritis center | n.d. | Cod liver oil, vinegar, alfalfa, yucca | n.d. | n.d. | n.d. | n.d. | Brown et al (abst) (17) |
| Mexico (Jalisco) | 2001 | 103 | Outpatients of a teaching hospital in Mexico | 54% | n.d. | 59%a | 12%a | n.d. | n.d. | Aceves-Avila et al (15) |
| USA (NM) | 2004 | 95 | Patients of 6 primary care clinics at University of New Mexico; 49.2% hispanic, 50.8% white | 60% | Glucosamine, chondroitin, garlic, fish oil, flaxseed oil, vinegar, ginger | n.d. | n.d. | 70%b | n.d. | Herman et al (24) |
| USA (NC) | 2009 | 192 | Family practice and private rheumatology patients in North Carolina, 2 seprately analyzed subgroups (1=Primary care and 2=Specialist clinic), ~ 25% African American | 31% | Glucosamine, chondroitin, fish oil, garlic, MSM, shark cartilage, cod liver oil, aloe vera, flaxseed oil, ginger | n.d. | n.d. | n.d. | n.d. | Callahan et al (19) |
| USA (NY) | 2010 | 166 | Patients seen by rheumatologist in tertiary care center in NY | 52% | Glucosamine, chondroitin, green tea, ginkgo biloba, echinacea, gin-soaked raisins | n.d. | n.d. | n.d. | n.d. | Efthimiou et al (23) |
| USA (Multi-Regional) | 2012 | 11970 | CORONA registry; private rheumatology clinic patients throughout the USA | 35% | Fish Oil | n.d. | n.d. | n.d. | n.d. | Pepmueller et al (abst) (36) |
| USA (PA) | 2013 | 102 | Rheumatology clinic patients | 58% | Green tea, fish oil, glucosamine, chondroitin | n.d. | n.d. | 26%b | n.d. | DiRenzo et al (abst) (22) |
| USA (AL, GA, NC, SC, MI) | 2014 | 855 | CLEAR registry, African Americans with diagnosis of RA | n.d. | Fish oil, garlic, glucosamine and/or chondroitin,vinegar, gin-soaked raisins | n.d. | n.d. | n.d. | n.d. | Tamhane et al (32) |
| USA (NY) | 2015 | 84 | Individuals with RA in New York, NY | 31% | n.d. | n.d. | n.d. | n.d. | n.d. | Ward et al (abst) (35) |
| EUROPE | ||||||||||
| Ireland (Dublin) | 1983 | 100 | Patients attending rheumatology department of hospital | n.d. | Cod liver oil, green-lipped sea mussel extract, vinegar | n.d. | n.d. | n.d. | n.d. | Cassidy et al (20) |
| UK (Birmingham) | 1983 | 199 | Patients in rheumatology department of hospital | 41% | Green mussel, cod liver oil | 21% | n.d. | n.d. | n.d. | Struthers et al (31) |
| UK (Salford) | 1983 | 94 | Patients receiving treatment in a hospital-based rheumatology clinic | 59% | Comfrey tea, ginseng, devil’s claw, kelp, green lipped sea mussel, garlic | 23% | n.d. | n.d. | n.d. | Higham et al (25) |
| Turkey (Ankara) | 2014 | 279 | Rheumatology unit patients of 10 university and 4 state hospitals in Turkey | 55% | n.d. | 73% | 10%b | 4%b | Tokem et al (33) | |
| OCEANA | ||||||||||
| Australia (Melbourne) | 1985 | 90 | Patients of an outpatient teaching clinic | n.d. | Cod liver oil, vinegar, kelp tablets, lecithin | n.d. | n.d. | n.d. | 80% | Kestin et al (37) |
| Australia (Melbourne) | 2002 | 101 | Patients who attended a private rheumatology practice | 44% | Fish oil, ginger, celery seed extract, green-lipped sea mussel extract | n.d. | 4% | n.d. | 90% | Buchbinder et al (18) |
| ASIA | ||||||||||
| Malaysia (Selangor) | 2002 | 77 | Patients from 3 rheumatology clinics in Klang Valley | 69%a | n.d. | 63%a | 16%a | n.d. | n.d. | Chow et al (21) |
| South Korea (Seoul) | 2003 | 173 | Patients from 2 rheumatology clinics affiliated with a Korean university hospital | 46%a | n.d. | 33%a,b | 9%a,b | 32%a,b | Kim et al (28) | |
| Japan (Tokyo) | 2006 | 3815 | Patients from the Institute of Rheumatology, Tokyo Women’s Medical University in Tokyo, Japan | 23% | Ginger, propolis, chlorella, immune milk, chondroitin, royal jelly | n.d. | n.d. | n.d. | n.d. | Kajiyama et al (27) |
| South Korea (Iksan) | 2008 | 153 | Rheumatology outpatients in 2 university hospitals in Jeonbuk, Korea | 70% | n.d. | 49%b | 15%b | 24%b | 97%b | Lee et al (29) |
| Japan (Beppu) | 2009 | 296 | University and private rheumatology clinics in Oita prefecture, Japan | 61% | Cartilage, glucosamine, mushroom products, fish oil, chondroitin | 35%b | n.d. | 13%b | 100%b | Ikuyama et al (26) |
| Philippines (Quezon) | 2010 | 32 | Rheumatology clinic patients of 2 tertiary care centers | 53% | n.d. | n.d. | n.d. | n.d. | 88% | Vista et al (abst) (34) |
| Malaysia (Putrajaya) | 2012 | 75 | Rheumatology clinic patients of Putrajaya Hospital, Malaysia | 41% | Glucosamine, fish oil | n.d. | n.d. | n.d. | n.d. | Mohd Noor et al (abst) (30) |
| MIDDLE EAST | ||||||||||
| Lebanon (Beirut) | 2012 | 168 | Patients of 3 hospital-based rheumatology clinics in Beirut | 19%a | Extraborojo, Herborem, Antiflam, Rheuma Golden Feel | 64%a | 24%a,b | 40%a,b | n.d. | Alaaeddine et al (16) |
n.d. = not described.
a = value describes a subgroup of patients in which >50% but <100% were RA patients
b = value describes a subgroup of patients in which >50% but <100% were NDS users (as apposed to users of other forms of CAM)
The majority of the studies (n = 20) were conducted in an RA-specific population or had separate data analyses for an RA subgroup: Three studies did not have a separate RA data analysis, but greater than 50% of the study population were patients with RA (16, 21, 28). Most studies recruited volunteers from specialty rheumatology clinics and/or from primary care clinics, with the exception of one study that did not specify the participant recruitment site (35). Most studies verified the RA diagnosis from the provider or from chart review (n = 15). Of the eight studies that did not specify how the RA diagnosis was established (16, 17, 20, 25, 30, 35–37), all but one had populations obtained from a clinic or hospital setting (35). One study included self-reported RA patients in addition to those with provider-verified RA diagnosis (19). Most studies acquired their data via an in-person questionnaire based interview (12 of 23), two were self-administered surveys (22, 30), one was a telephone interview, two were mailed (19, 27) and six did not specify (15, 17, 26, 33, 36, 37). Five of the studies provided details of the questions asked or provided a copy of the survey (15, 18, 21, 27, 28). Only seven studies documented inclusion of an open-ended option to report supplements that were not pre-specified (15, 18, 21, 24, 27, 28, 37). Thus, in the majority of studies, insufficient details were provided to assess (and thus compare) the range of products queried. The specific definition of NDS varied greatly between studies, with descriptions including “nutraceutical”, “dietary supplements”, “food supplement”, “dietary substances”, “nutritional supplements”, “plant and animal derived OTC products” and “non-prescribed oral treatment”. Sixteen studies reported data on the prevalence and use of specific substances (16–20, 22–27, 30, 31, 37, 38), with 19 studies providing data on the overall prevalence of NDS use (Table 1). Many of the studies (n = 11) were published more than a decade prior to this review. Studies from 11 countries were identified (Table 1), representing the Americas (n = 9, primarily the US), Europe (n = 4, primarily the United Kingdom and Ireland), Oceana (n = 2 Australia), Asia (n = 7) and the Middle East (n = 1), with median sample size of n = 153 (range 32–11,970, mean = 852). The majority of studies (n = 15) were conducted in non-US countries and included data from n = 5,755 participants. In comparison, US studies (n = 7) reported data on n = 1,878 participants, if one excludes a single US study, published only in abstract form, reporting limited results on 11,970 participants (36).
Overall Prevalence and Characteristics of NDS Use (Table 1)
The average prevalence of NDS use reported by individuals with RA was 47% (median 52, range 19–70), with a similar average prevalence in studies conducted in the US (45%) vs. non-US countries (49%) or other specific regions (e.g. Asia, 52%). The highest rate of NDS use (70.4%) was reported by Lee (29) in South Korea. The lowest rate of NDS use (19.2%) was reported by Alaaeddine (16) in Lebanon. Of the 9 studies querying NDS efficacy for symptom management, an average of 47% of patients (range 21–73%) found NDS to be effective (15, 16, 21, 25, 26, 28, 29, 31, 33). Decreased pain intensity, improved sleep, alleviation of symptoms, health promotion, reduced swelling, reduced fatigue and improved activity level were some of the criteria patients used to gauge effectiveness. Seven studies reported the prevalence of patients experiencing adverse effects with an average prevalence of 13% (range 4–24%) (15, 16, 18, 21, 28, 29, 33). Examples of adverse effects included belching, gastrointestinal upset, itching, swelling, increased pain, headaches, low stamina, heartburn, drowsiness, nausea, vomiting, “allergy”, bruising, diarrhea and dermatologic manifestations. Of the seven studies that asked patients if they informed their healthcare providers about NDS use, the average prevalence of reported NDS usage was 30% (range 4–70%) (16, 22, 24, 26, 28, 29, 33). Common reasons for not reporting use included a perception that NDS were safe and that their physician did not ask. On average, 91% of patients across five studies capturing concurrent medication use reported taking their conventional prescribed RA treatment regimen concurrent with NDS use (18, 26, 29, 34, 37).
Reported Use of Specific NDS
Of the 16 studies reporting data on the prevalence of use of specific NDS products, marine-derived oils, glucosamine, chondroitin, vinegar, garlic and green-lipped sea mussel extract were most frequently ranked among top products used (Figure 2). When the average prevalence of use across studies for supplements reported in more than 1 study was assessed (Figure 3), green tea had the highest prevalence, although its use was only reported in 2 studies. NDS products with the highest prevalence of use, which were also the most frequently cited in studies spanning multiple regions of the world, included marine oils (17.3%), glucosamine (16.5%), vinegar (13.8%) and chondroitin (9.1%). Reported usage of certain NDS products appeared to be regionally or culturally-specific, such as: 1) the use of bee-derived products (propolis, royal jelly) and colostrum (immune milk) in Japan (Figure 3, stippled bars); 2) the use of green-lipped sea mussel extracts in the UK, Ireland, and Australia (Figure 3, shaded bars); and 3) the use of methylsulfonylmethane (MSM) and gin-soaked raisins in the United States (Figure 3, cross-hatched bars).
Figure 2.
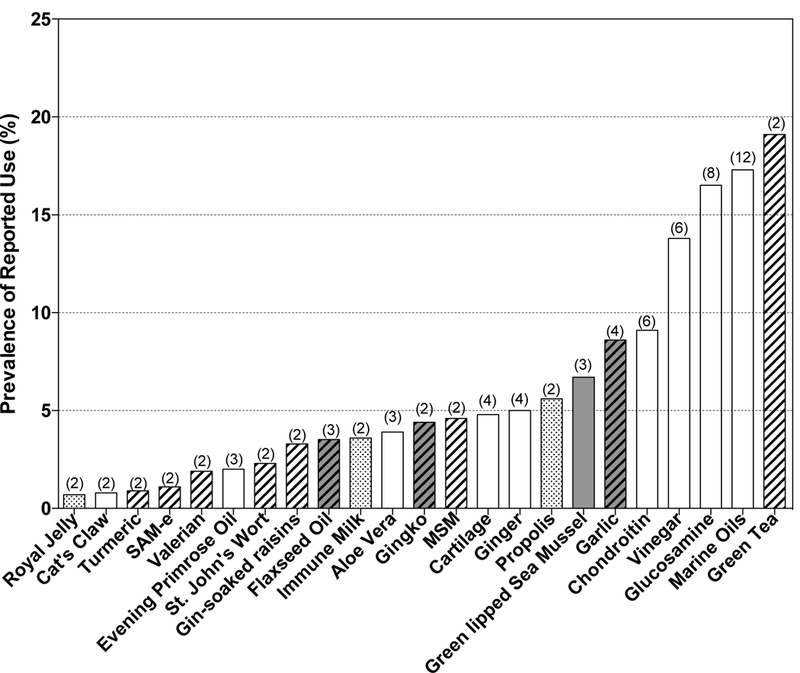
Specific NDS products reported in any study as being one of the 5 most commonly used NDS. The number of studies (n = 16 total) reporting high usage for each product is indicated. Proprietary products were not included.
Figure 3.
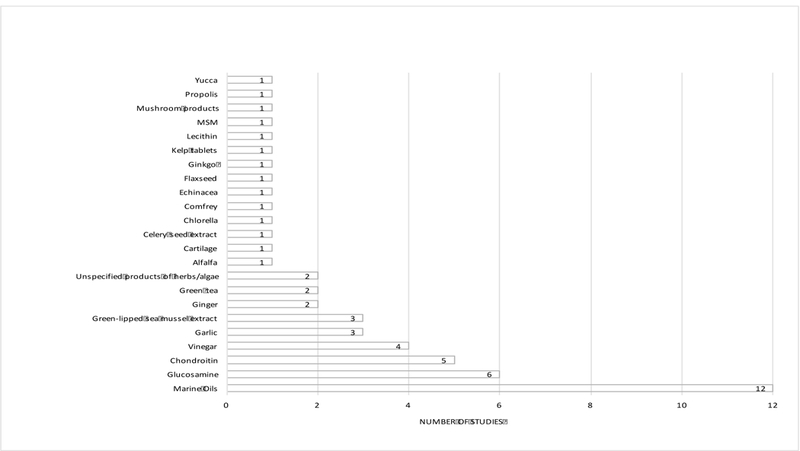
Specific NDS products with usage reported in > 1 study (n = 16 studies total) and their average prevalence of use in these studies are indicated. Number in parentheses indicates the number of studies reporting prevalence data that were included in the calculation. Bars with stippled fill indicate products only used in Japan. Bars with cross hatching indicate products with reported usage only in the US. Shaded bar indicates product with reported usage only in the U.K. and Commonwealth countries. Shaded bars with cross hatching indicates products with reported usage only in US and UK/Commonwealth countries.
DISCUSSION
Given the potentially higher risk of NDS-associated side effects in individuals with RA due to interactions with conventional treatments or systemic manifestations of the disease, it is notable that data from this scoping review identified a high prevalence of NDS use by RA populations world-wide. Similarly, as this review also confirms a frequent lack of disclosure of NDS use by individuals with RA, as has been reported for adults in the general population, our goal of identifying population-based patterns of specific product use for RA appears to be clinically relevant.
Significant heterogeneity in methodology and the definition of NDS between studies limited the ability to make strict comparisons of prevalent NDS use across studies and, therefore, countries, thus supporting the use of a scoping review strategy. For example, vitamins and minerals were included in overall NDS use in a minority of studies (26), while in other studies, use of herbal remedies or traditional forms of herbal medicine were reported separately from NDS (16, 21, 24, 27, 28, 33). Interestingly, categorization of specific NDS also varied greatly across studies. For example, garlic extract was variously categorized as an “herb taken orally” (23) vs. a “dietary supplement” distinct from herbal (27), while a minority of studies classified marine oils as a “diet” or “dietary substance” separately from herbs and supplements (25, 35). Despite these limitations, data summarized here clearly demonstrate that: 1) US RA patients use NDS at a higher rate than the general population (e.g. 31–60% prevalence in US RA population vs. 18% of US adults(1)) and that 2) a high average prevalence of RA NDS usage globally (47%) is reflective of very similar rates of NDS use across countries, despite different cultural traditions related to medicinal herb use.
Significant variances did exist, however, as to the specific NDS products used, which were suggestive of geographic and/or cultural patterns. While certain NDS products, such as marine oils, maintained a high rate of use across countries and time periods, other products appeared to have regional and/or temporal differences in patterns of use. For example, use of bee-derived products appeared to be specific to Japan, while green-lipped sea mussel extract was only reported in studies from Australia, Ireland and the U.K. Of note, however, all studies documenting use of green-lipped sea mussel extract were also conducted more than a decade ago, making it difficult to ascertain the relative importance of cultural vs. temporal trends in patterns of use for this NDS product. Even when assessing US studies, which were distributed across time, differences in study population demographics precluded an accurate assessment of temporal changes in patterns of NDS use; for example, older studies tended to be from the western US, while most contemporary US studies were conducted in the northwest or south in populations that also differed by race. In any case, given recent overall increases and changing trends in US sales of specific NDS, such as turmeric, (4, 39) it is possible that current patterns of NDS use in RA, at least in the US, may not be well reflected by the totality of available data reviewed here.
Methodologic differences in data collection may have also accounted for some differences in patterns of reported NDS use between studies, including the use of pre-determined lists of supplement options for participants to choose from and/or the lack of an open-ended response option, which was the case in the majority of the studies in this review. Other elements of study design that limit conclusions to be drawn include: recruitment of distinct sample groups (e.g. majority were limited to those seeking treatment in rheumatology clinics) that may not have been representative of the overall RA population; risk of bias due to attrition of volunteers not taking NDS; and use of in-person interviews, a methodology used for a majority of studies that may have impacted subject responses.
NDS use in an RA population could have particularly significant consequences; a high potential for drug-NDS interactions exists given the intensive pharmaceutical management of this population, for both RA itself and for common associated co-morbidities such as cardiovascular disease. Reported rates of NDS-related adverse events varied across studies in this review. However, the mean rate of adverse events in this review (13%), despite having a small sample of studies reporting this statistic, was similar to results reported in a recent survey of DS use by the general population in Japan (9%) (40). A review of potential herb-drug interactions in a general population found that 40% of herbal medicine users had a potential herb-drug interaction with one of their prescribed medications (5). Some specific NDS frequently used by RA patients have documented drug interactions. For example, fish oils, glucosamine and ginger have been reported to increase INR in patients taking warfarin (41). Glucosamine has been reported to affect glycemic control (41) and evening primrose oil may lower seizure threshold (41). Specific drug interactions between NDS frequently used by RA patients and anti-rheumatic therapies are largely unknown.
While 47% of RA patients taking NDS reported beneficial effects, evidence of efficacy for most NDS with high prevalent use in this review was lacking. Of the five most commonly reported NDS documented here, only marine oil supplements (fish or cod liver oil) have been extensively studied and demonstrated, in a systemic review and meta-analysis, to have a favorable effect on RA pain based on evidence of moderate quality (42). Marine oils have also been reported to improve multiple cardiovascular risk factors in RA patients and to reduce NSAID use for RA symptoms by 75% (43). A Cochrane systematic review of the effectiveness of herbal therapies in the treatment of RA determined that there was a potential benefit for two NDS, both of which were found to have a low prevalence of use in this review: 1) gamma linolenic acid (GLA), present in primrose oil, borage seed oil and blackcurrant seed oil (44) and 2) thunder god vine, which was found to be effective in relieving symptoms in a small number of studies but was associated with several side effects, including effects on male fertility, dysmenorrhea, renal dysfunction and potential teratogenicity (44, 45). Unfortunately, many of the trials were limited by design flaws (44). A placebo-controlled study investigating the potential utility of glucosamine, an NDS commonly used for RA worldwide, concluded that while there was no evidence of an anti-rheumatic effect in RA, as determined by tender and swollen joint counts, ESR or CRP levels, glucosamine did provide some symptomatic benefit, as assessed by both patients and physicians (46).
In addition to the relative lack of efficacy data and some reporting of adverse events, a significant concern related to the high prevalence of NDS use in RA is the communication barrier that exists between patients and their physicians regarding their NDS use. A study surveying patients with various joint disease found that 92% of patients preferred a physician who informed them about alternative therapies; however 30% did not discuss CAM use with their physician and 21% refrained from this discussion because they knew their doctor would not approve of their use (47). This is consistent with the average number of patients with RA reporting that they informed their physicians in our review (30%). It appears patients may be correct in their assessment—a survey of rheumatologists on the subject ascertained that only 33% of American College of Rheumatology (ACR) rheumatologists felt they had enough knowledge of herbal medicines to discuss their use with patients and only 19% considered them part of legitimate medical practice (48).
Because the significant communication-gap between patients and their providers regarding NDS use could potentially expose patients to increased risk of harm, up to date assessments of RA population-based usage patterns may assist providers in remaining cognizant of their patients’ practices and enhance their ability to identify NDS-related clinical outcomes. This is particularly important in the US where federal oversight of NDS is primarily limited to an assessment of adverse events reported post-marketing. A continued increase and changing trends in the sale of specific NDS suggest that current US-based usage patterns may not be well captured in the disparate studies reported here, requiring a larger-scale, multiregional and non-provider affiliated survey to identify more current estimates of the prevalence and pattern of NDS use in the general RA population.
Supplementary Material
SIGNIFICANCE AND INNOVATIONS.
The prevalence of natural product dietary supplement (NDS) use by individuals with RA averages 47% worldwide, and is similar in the United States (US) vs. other countries, including those with stronger traditions of medicinal botanical use.
In a majority of cases, reported NDS usage in RA occurred concomitantly with use of pharmaceutical agents (91%) and was not reported to health care providers (70%).
Of the most common NDS reported to be used worldwide (marine oils, glucosamine, chondroitin and vinegar), only marine oils have a moderate evidence base demonstrating efficacy in RA.
Because NDS use by individuals with RA is under reported but common and lacks a strong evidence base for efficacy or safety in this population, an awareness of population-based NDS usage patterns may help to optimize the delivery of RA care and inform future research efforts.
ACKNOWLEDGEMENTS
This research was supported by the National Center for Complementary and Integrative Health (NCCIH) at the National Institutes of Health (R34AT007837 to JLF). KH received support from the NIH National Heart, Lung and Blood Institute (T35HL007479). Views expressed here do not necessarily represent those of the NIH.
This work was supported by the National Center for Complementary and Integrative Health (NCCIH) at the National Institutes of Health (R34AT007837 to JF).
Footnotes
CONFLICT OF INTEREST None of the authors have any conflict of interest related to the work reported here.
REFERENCES
- 1.Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002–2012. Natl Health Stat Report. 2015(79):1–16. [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008(12):1–23. [PubMed] [Google Scholar]
- 3.Wilson PB. Dietary supplementation is more prevalent among adults with arthritis in the United States population. Complement Ther Med. 2016;29:152–7. [DOI] [PubMed] [Google Scholar]
- 4.Smith TKEV, Johnson J. Sales of herbal dietary supplements in US increased 7.5% in 2015 consumers spent $6.92 billion on herbal supplements in 2015, marking the 12th consecutive year of growth. HerbalGram. 2016;111:67–73. [Google Scholar]
- 5.Bush TM, Rayburn KS, Holloway SW, Sanchez-Yamamoto DS, Allen BL, Lam T, et al. Adverse interactions between herbal and dietary substances and prescription medications: a clinical survey. Altern Ther Health Med. 2007;13(2):30–5. [PubMed] [Google Scholar]
- 6.Tarn DM, Paterniti DA, Good JS, Coulter ID, Galliher JM, Kravitz RL, et al. Physician-patient communication about dietary supplements. Patient Educ Couns. 2013;91(3):287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh JA, Wells GA, Christensen R, Tanjong Ghogomu E, Maxwell L, Macdonald JK, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011(2):CD008794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Attar SM. Adverse effects of low dose methotrexate in rheumatoid arthritis patients. A hospital-based study. Saudi Med J. 2010;31(8):909–15. [PubMed] [Google Scholar]
- 9.Shafrin J, Ganguli A, Gonzalez YS, Shim JJ, Seabury SA. Geographic Variation in the Quality and Cost of Care for Patients with Rheumatoid Arthritis. J Manag Care Spec Pharm. 2016;22(12):1472–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daudt HM, van Mossel C, Scott SJ. Enhancing the scoping study methodology: a large, inter-professional team’s experience with Arksey and O’Malley’s framework. BMC Med Res Methodol. 2013;13:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arksey H O’Malley L. Scoping studies: towards a methodological framework. International journal of social research methodology. 2005;8(1):19–32. [Google Scholar]
- 12.Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. [DOI] [PubMed] [Google Scholar]
- 14.Groff R, Strom M, Hopkins L, Feng L, Hopkins A, Funk J. Dietary supplements and nutritional approaches used for rheumatoid arthritis self-management. FASEB Journal. 2017;31(1). [Google Scholar]
- 15.Aceves-Avila FJ, Medina F, Fraga A. Herbal therapies in rheumatology: The persistence of ancient medical practices. Clinical and Experimental Rheumatology. 2001;19(2):177–83. [PubMed] [Google Scholar]
- 16.Alaaeddine N, Okais J, Ballane L, Baddoura RM. Use of complementary and alternative therapy among patients with rheumatoid arthritis and osteoarthritis. Journal of Clinical Nursing. 2012;21(21–22):3198–204. [DOI] [PubMed] [Google Scholar]
- 17.Brown JH SP, Fries JF. Unorthodox treatments in rheumatoid arthritis. Arthritis Rheum. 1980;23(suppl 1):657–8. [Google Scholar]
- 18.Buchbinder R, Gingold M, Hall S, Cohen M. Non-prescription complementary treatments used by rheumatoid arthritis patients attending a community-based rheumatology practice. Internal Medicine Journal. 2002;32(5–6):208–14. [DOI] [PubMed] [Google Scholar]
- 19.Callahan LF, Wiley-Exley EK, Mielenz TJ, Brady TJ, Xiao C, Currey SS, et al. Use of complementary and alternative medicine among patients with arthritis. Preventing Chronic Disease. 2009;6(2):A44. [PMC free article] [PubMed] [Google Scholar]
- 20.Cassidy M JA, Bresnihan B. The use of unproven remedies for rheumatoid arthritis in Ireland . Irish Med J. 1983;76:464–5. [PubMed] [Google Scholar]
- 21.Chow SK, Yeap SS, Goh EML, Veerapen K, Lim KKT. Traditional medicine and food supplements in rheumatic diseases. Medical Journal of Malaysia. 2002;57(3):283–91. [PubMed] [Google Scholar]
- 22.DiRenzo D, Sun H, Kirchner HL, Newman ED. Complementary and alternative medicine in rheumatoid arthritis-persistently high use over the past decade despite advent of biologics. Arthritis and Rheumatism. 2013;65:S959. [Google Scholar]
- 23.Efthimiou P, Kukar M, Mackenzie CR. Complementary and alternative medicine in rheumatoid arthritis: no longer the last resort! HSS Journal. 2010;6(1):108–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herman CJ, Allen P, Hunt WC, Prasad A, Brady TJ. Use of complementary therapies among primary care clinic patients with arthritis. Preventing Chronic Disease. 2004;1(4):A12. [PMC free article] [PubMed] [Google Scholar]
- 25.Higham C AC, Jayson MIV. Non-prescribed treatments in rheumatic diseases. Practitioner. 1983;227:1201–5. [PubMed] [Google Scholar]
- 26.Ikuyama S, Imamura-Takase E, Tokunaga S, Oribe M, Nishimura J. Sixty percent of patients with rheumatoid arthritis in Japan have used dietary supplements or health foods. Modern Rheumatology. 2009;19(3):253–9. [DOI] [PubMed] [Google Scholar]
- 27.Kajiyama H, Akama H, Yamanaka H, Shoji A, Matsuda Y, Tanaka E, et al. One third of Japanese patients with rheumatoid arthritis use complementary and alternative medicine. Modern Rheumatology. 2006;16(6):355–9. [DOI] [PubMed] [Google Scholar]
- 28.Kim HA, Seo YI. Use of complementary and alternative medicine by arthritis patients in a university hospital clinic serving rheumatology patients in Korea. Rheumatology International. 2003;23(6):277–81. [DOI] [PubMed] [Google Scholar]
- 29.Lee MS, Lee MS, Yang CY, Lee SI, Joo MC, Shin BC, et al. Use of complementary and alternative medicine by rheumatoid arthritis patients in Korea. Clinical Rheumatology. 2008;27(1):29–33. [DOI] [PubMed] [Google Scholar]
- 30.Mohd Noor N, Jenm DH, Hussein H, Mohd Isa L, Shahril NS. Influence of diet in inducing flares and use of dietary supplement in patients with rheumatoid arthritis. International Journal of Rheumatic Diseases. 2012;15:145. [Google Scholar]
- 31.Struthers GR SD, Scott DGI. The use of alternative treatments by patients with rheumatoid arthritis. Rheumatol Int. 1983;3:151–2. [DOI] [PubMed] [Google Scholar]
- 32.Tamhane A, McGwin G Jr, , Redden DT, Hughes LB, Brown EE, Westfall AO, et al. Complementary and alternative medicine use in African Americans with rheumatoid arthritis. Arthritis care & research. 2014;66(2):180–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tokem Y, Parlar Kilic S, Ozer S, Nakas D, Argon G. A multicenter analysis of the use of complementary and alternative medicine in Turkish patients with rheumatoid arthritis: holistic nursing practice review copy. Holistic Nursing Practice. 2014;28(2):98–105. [DOI] [PubMed] [Google Scholar]
- 34.Vista ES, Hamijoyo LA, Navarra SV. Survey on the use of complementary therapies among Filipinos with arthritis. International Journal of Rheumatic Diseases. 2010;13:230.20704619 [Google Scholar]
- 35.Ward B, Thomas L, Kiely M, Yazici Y, Woolf K. Use of dietary supplements in individuals with rheumatoid arthritis. FASEB Journal. 2015;29(1). [Google Scholar]
- 36.Pepmueller PH, Jandali R, Sharma A, Grant S, Saunders KC. Use and long term use of complementary and alternative medicine in rheumatoid arthritis patients. Arthritis and Rheumatism. 2012;64:S164–S5. [Google Scholar]
- 37.Kestin M MM, Miller L, Littlejohn G, Wahlgvist M. The use of unproven remedies for rheumatoid arthritis in Australia. Med J Aust. 1985;143:516–8. [DOI] [PubMed] [Google Scholar]
- 38.Huilgol VR HC, Higgins MJ, Alton J, Tilly SJ, Harris RD, Brooks PM. Non-traditional medication for rheumatoid arthritis. Aust NZ Med J. 1982;12:562. [Google Scholar]
- 39.Ernst E Usage of complementary therapies in rheumatology: a systematic review. Clin Rheumatol. 1998;17(4):301–5. [DOI] [PubMed] [Google Scholar]
- 40.Chiba T, Sato Y, Kobayashi E, Ide K, Yamada H, Umegaki K. Behaviors of consumers, physicians and pharmacists in response to adverse events associated with dietary supplement use. Nutr J. 2017;16(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao S, Otieno F, Akpan A, Moots RJ. Complementary and Alternative Medicine Use in Rheumatoid Arthritis: Considerations for the Pharmacological Management of Elderly Patients. Drugs Aging. 2017;34(4):255–64. [DOI] [PubMed] [Google Scholar]
- 42.Senftleber NK, Nielsen SM, Andersen JR, Bliddal H, Tarp S, Lauritzen L, et al. Marine Oil Supplements for Arthritis Pain: A Systematic Review and Meta-Analysis of Randomized Trials. Nutrients. 2017;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cleland LG, Caughey GE, James MJ, Proudman SM. Reduction of cardiovascular risk factors with longterm fish oil treatment in early rheumatoid arthritis. J Rheumatol. 2006;33(10):1973–9. [PubMed] [Google Scholar]
- 44.Cameron M, Gagnier JJ, Chrubasik S. Herbal therapy for treating rheumatoid arthritis. Cochrane Database Syst Rev. 2011(2):CD002948. [DOI] [PubMed] [Google Scholar]
- 45.Macfarlane GJ, El-Metwally A, De Silva V, Ernst E, Dowds GL, Moots RJ, et al. Evidence for the efficacy of complementary and alternative medicines in the management of rheumatoid arthritis: a systematic review. Rheumatology (Oxford). 2011;50(9):1672–83. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura H, Masuko K, Yudoh K, Kato T, Kamada T, Kawahara T. Effects of glucosamine administration on patients with rheumatoid arthritis. Rheumatol Int. 2007;27(3):213–8. [DOI] [PubMed] [Google Scholar]
- 47.Jong MC, van de Vijver L, Busch M, Fritsma J, Seldenrijk R. Integration of complementary and alternative medicine in primary care: what do patients want? Patient Educ Couns. 2012;89(3):417–22. [DOI] [PubMed] [Google Scholar]
- 48.Berman BM, Bausell RB, Lee WL. Use and referral patterns for 22 complementary and alternative medical therapies by members of the American College of Rheumatology: results of a national survey. Arch Intern Med. 2002;162(7):766–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


