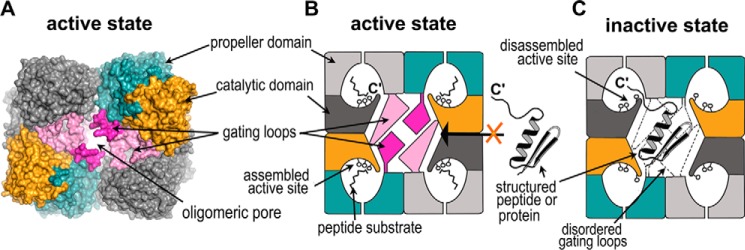
Figure 9.
Substrate-screening mechanism in S9Cdr carboxypeptidase. A, surface representation of the tetramer of the active enzyme showing the gate of the enzyme formed by the oligomerization of the monomers. The gating loops are colored in magenta and light pink, and rest of the color scheme of subunits follows Fig. 5A. B, schematic of the active state of the enzyme wherein the gating loops are ordered and the active site is assembled. It is conducive for cleavage of smaller peptides but prevents the entry of larger peptides or proteins into the active site cavity. C, schematic of the inactive state of the enzyme in which the gating loops are disordered and the active site is disassembled. The structured peptides or proteins may enter through the oligomeric pore and their C′-end may have access to the active site cavity via the side opening of the monomer, but they cannot be hydrolyzed due to the disassembled active site.