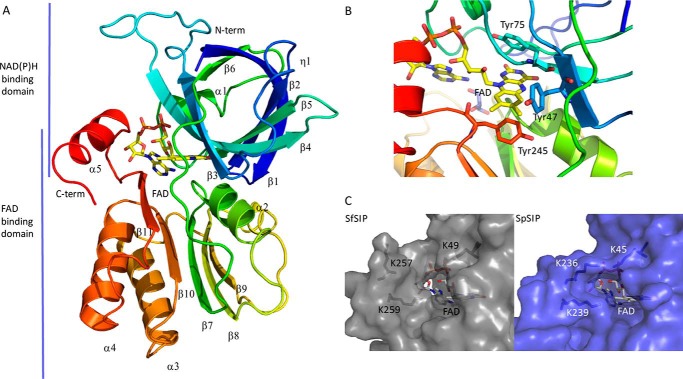
Figure 1.
Structural characterization of SIP. A, overall structure of SIP; B, close-up of the FAD pocket and stacking residues (Tyr-47, Tyr-75, and Tyr-245); C, putative NAD(P)H-binding pocket: comparison of the C-terminal helix of SIPs from S. frigidimarina (SfSIP) and S. putrefaciens (SpSIP). Lys-236 from SpSIP seems to make a bridge across the pocket, whereas Lys-257 in SfSIP is more distant from the isoalloxazine ring and pointing outwards from the pocket making more space available for substrate binding (ferric-siderophore and/or electron donors).