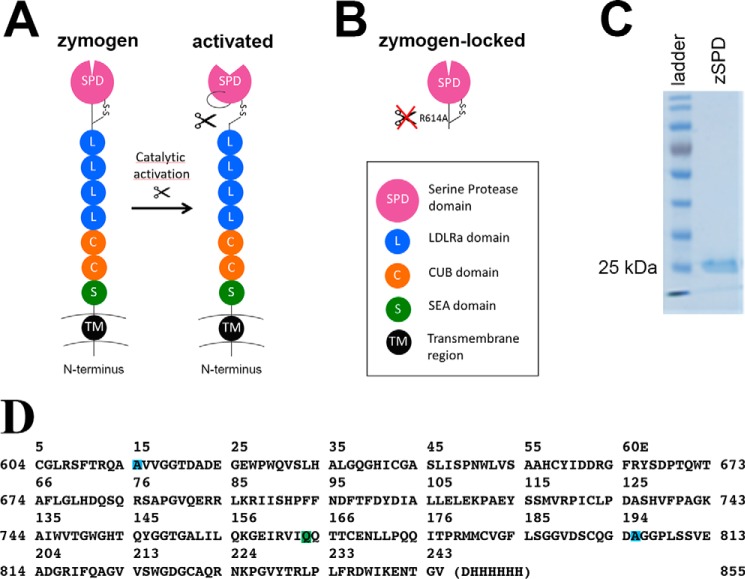
Figure 1.
Matriptase protein and constructs. A, schematic drawing of full-length matriptase with the N-terminal transmembrane region (black), the SEA domain (green), two CUB domains (orange), four LDLRa domains (blue), and the C-terminal serine protease domain (purple). The cleaved activation loop rearranges to create the catalytically active protease. B, schematic drawing of the zymogen-locked form of matriptase characterized in this paper, consisting of only the serine protease domain with position R614A mutated to avoid activation (zSPD). C, a Coomassie-stained SDS-PAGE (10%) shows that zSPD (consisting of only the serine protease domain, as shown in B) is pure and the expected size (25–30 kDa). D, the sequence of the crystallized form of zSPD (zSPD-S805A) with an additional mutation in the active site, shown with matriptase numbering (sides) and chymotrypsin numbering (top). The mutations are color-coded: R614A (blue), N772Q (green), and S805A (blue).