Figure 6.
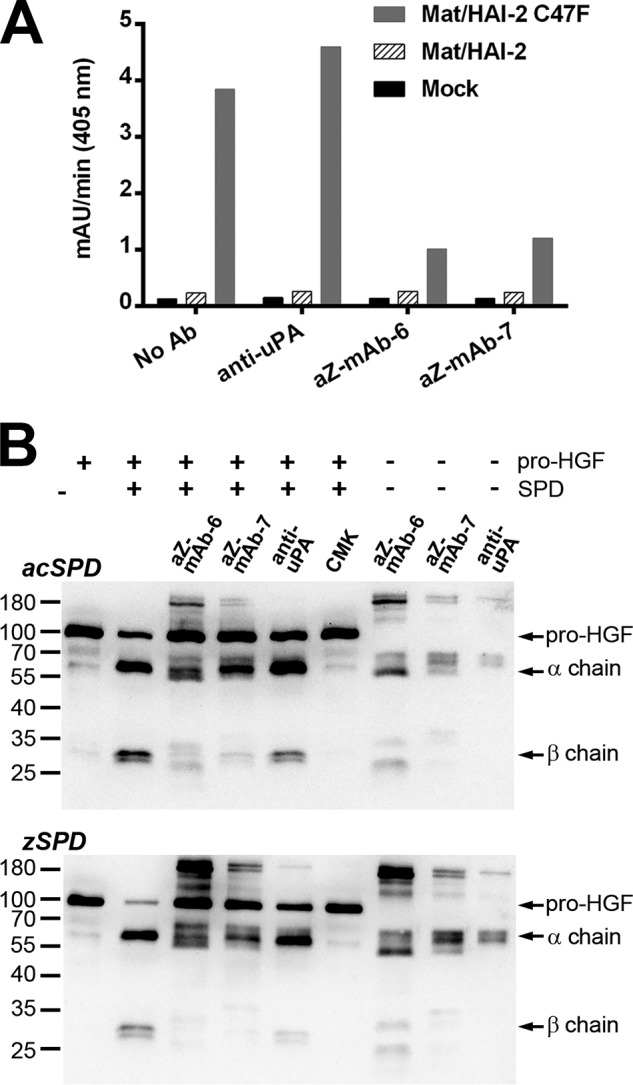
A, the monoclonal antibodies aZ-mAb-6 and -7 inhibit matriptase activity in the protein extracts of transiently transfected cells. HEK293 cells were transiently transfected with empty expression vector (mock) or expression vectors encoding WT full-length matriptase together with WT HAI-2 or mutant HAI-2 C47F, respectively. Protein extracts were preincubated for 1 h at room temperature with 500 ng of antibody (anti-uPA, aZ-mAb-6, aZ-mAb-7, or no antibody), and a peptidolytic activity assay was subsequently carried out with 300 μm chromogenic substrate S-2288. The absorbance of the reaction mixture was measured at 405 nm continuously every 5 min for 5 h, and the mean velocity of the substrate reaction (in milli-absorbance units/min) was calculated. The figure shown is representative of three replicates. B, aZ-mAb-6 and -7 inhibit matriptase-mediated pro-HGF cleavage. Pro-HGF (60 nm final concentration) was incubated for 4.5 h at 37 °C with either 200 nm zSPD or 1 nm acSPD that had been preincubated with aZ-mAb-6, aZ-mAb-7, anti-uPA, or biotin-RQRR-CMK, as indicated above the gels. The processing of pro-HGF was visualized on Western blotting using an antibody that recognizes pro-HGF as well as its α and β chains. Antibody-only samples were prepared in parallel to show the cross-reactivity of the antibodies themselves with the secondary antibody.
