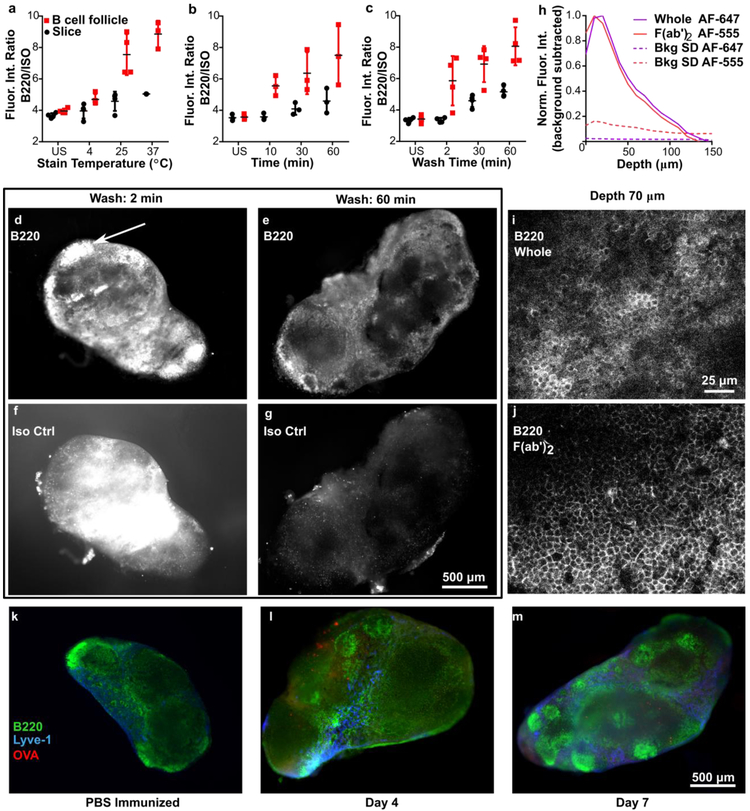
Figure 1.
Immunostaining procedure yields bright and specific staining at least 70 μm deep and reveals structures in lymph node, (a-c) Testing for sufficient stain time, temperature, and wash time. The fluorescent intensity ratio (FITC-anti-B220/ Alexa Fluor 594-IsoCtrl) was averaged over the entire slice or over a selected B cell follicular area, for stained and unstained (US) slices. Conditions for a: 1-hr stain, 30-min wash; b: 37 °C, 30-min wash; c: 37 °C, 1-hr stain. Each dot represents a single slice. Data from single experiment. Error bars denote standard deviation, (d-g) Representative images of 300-μm-thick lymph node slices stained with anti-B220 and its isotype control, after washing for 2 or 60 minutes. White arrow in (e) indicates a representative B cell follicle. Brightness and contrast were adjusted uniformly within each channel. (h) Fluorescent signal of anti-B220 IgG and F(ab’)2 fragment in a 300-μm-thick slice as measured by confocal microscopy. Dotted lines indicate one standard deviation (SD) of autofluorescence background as a function of depth, and solid colored lines indicate the average background-subtracted intensity (averaged across 20 positions). Data compiled from 2 replicate experiments, N = 20 z-stacks per condition. (i-j) Confocal images of antibody and fragment staining at a depth of 70 μm, both labeled with Alexa Fluor 647. Brightness and contrast were adjusted uniformly between the images, (k-m) Immunostaining of slices at different timepoints can reveal changes in substructure. After vaccination with rhodamine-OVA in CFA or with saline control, axillary LN slices were stained live immunostained with FITC-anti-B220 and eFluor 660-anti-Lyve-1. The images chosen were representative of the most common features observed in the majority of slices. Brightness and contrast were uniformly adjusted between images.