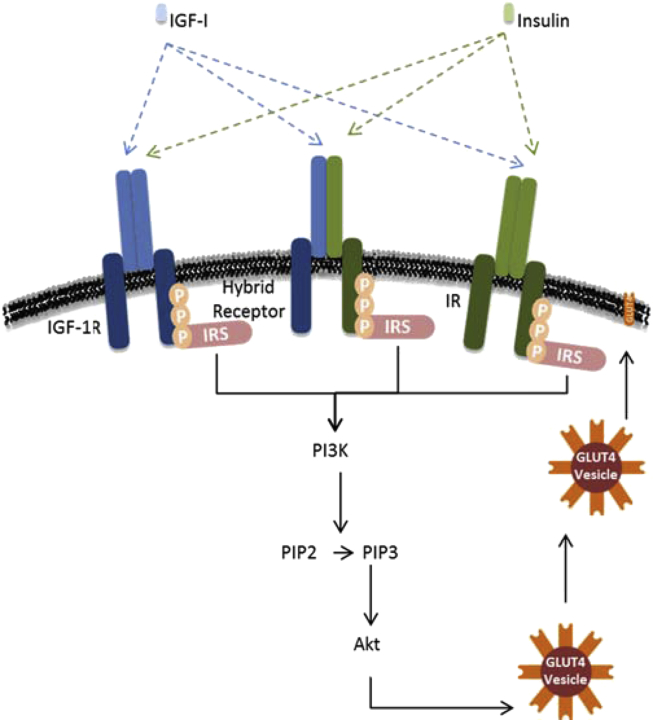
Figure 2.
– IGF-I/Insulin PI3K/AKT signaling pathway. Insulin elicits a diverse array of biological responses by binding to its specific receptor. The insulin receptor (IR) belongs to a subfamily of receptor tyrosine kinases that includes the IGF type 1 receptor (IGF-1R). These two receptors can form hybrids. These receptors are tetrameric proteins consisting of two alpha and two beta subunits that function as allosteric enzymes in which the alpha subunit inhibits the tyrosine kinase activity of the beta subunit. Once IGF-I or insulin is bound the receptor, autophosphorylation occurs. This provides docking sites for IRS (Insulin Receptor Substrates), which, in turn, are phosphorylated. Tyrosine-phosphorylated IRS then displays binding sites for numerous signaling partners. PI3K phosphorylates PIP2 to PIP3 (Phosphatidylinositol −3, 4, 5-Triphosphate). Akt possesses a domain that interacts directly with PIP3. Akt is required for insulin-stimulated glucose transport. GLUT4 (Glucose Transporter Protein-4) is ultimately translocated from an intracellular compartment to the plasma membrane, which results in increased glucose uptake.