Abstract
Background
Topical efinaconazole 10% solution is a promising new treatment for distal lateral subungual onychomycosis (DLSO). However, it is unknown whether this treatment is both compatible and efficacious in individuals wearing toenail polish.
Materials and Methods
We evaluated the efficacy and compatibility of efinaconazole 10% solution with concurrent nail polish use in treating DLSO over 52 weeks. Efficacy was assessed using the onychomycosis severity index (OSI) and by measuring nail growth and thickness, while compatibility with nail polish was evaluated with questionnaires.
Results
Eleven patients completed the study; 6 wore nail polish regularly and 5 abstained from polish. The efficacy of efinaconazole was not diminished by concurrent nail polish use as measured by OSI, nail growth, and thickness. However, this treatment produced undesirable cosmetic changes to the quality of nail polish over time.
Conclusions
While efinaconazole 10% solution is an effective treatment of DLSO in patients wearing nail polish, this treatment may diminish the quality of the polish. Further research and development is needed to enhance the compatibility of topical onychomycosis treatments with nail polish use.
Keywords: Efinaconazole, Distal lateral subungual onychomycosis, Nail polish, Onychomycosis severity index
Introduction
Distal lateral subungual onychomycosis (DLSO) is a commonly encountered dermatological disease. DLSO, which is caused by a variety of dermatophyte organisms, typically has an indolent and a chronic course. Traditional therapies for DLSO have been comprised of mainly oral antifungal medications; however, many of these treatments have side effect profiles that limit their applicability. Because of the potential toxicities associated with traditional oral therapies for DLSO, there has been great interest in finding safe and efficacious alternatives.
Topical efinaconazole 10% solution (Jublia), an FDA-approved treatment for DLSO, has been shown to be efficacious and well tolerated by patients [1]. In clinical trials, efinaconazole was applied daily to the nail plate, lateral and proximal nail folds, hyponychium, and undersurface of the nail. Systemic absorption and associated toxicities are minimized with this topically applied medication. Complete cure and mycologic cure rates using efinaconazole 10% solution were within the range of those seen with oral antimycotic medications, making this topical agent a welcome therapy for onychomycosis [2, 3].
Toenail polish is very popular among women, especially during summer months, and wearing toenail polish is currently a major limitation of topical efinaconazole for the treatment of DLSO. Few studies have evaluated the compatibility of topical onychomycosis therapy with concurrent toenail polish use [4, 5, 6]. In this study, we sought to evaluate the efficacy and compatibility of efinaconazole 10% solution for the treatment of toenail onychomycosis in patients wearing toenail polish compared to those without polish.
Methods
Study Population
This is a single-center, prospective, blinded, 52-week study conducted at a tertiary care academic center. This study was performed with the approval from the Institutional Review Board for Human Use of the University of Alabama, Birmingham.
Inclusion criteria consisted of being female and aged 19–70 years with DLSO effecting at least 1 great toenail. The target great toenail thickness was ≤3 mm. DLSO was diagnosed by having a positive potassium hydroxide microscopy and a culture of a dermatophyte. Exclusion criteria were a history of immunosuppression, uncontrolled diabetes mellitus, or psoriasis. Patients were also excluded if they had severe target toenail DLSO or 3 or more dermatophytomas on the target toenail. The patients were required to refrain from wearing gel- or plastic-based polishes that are used in salons and require curing.
Patients meeting the inclusion and exclusion criteria were enrolled consecutively over 11 months, resulting in a total of 13 enrolled patients. Patients who self-identified as frequently wearing toenail polish were placed in the study group, while the control group was comprised of women who agreed to abstain from wearing toenail polish throughout the length of the study. Patients with symptomatic tinea pedis at screening were treated with luliconazole cream for 2 weeks.
Because nail size is proportional to body size, with men having larger nails on average, men were excluded from the study since they would likely have been placed exclusively in the control group and thus introduced gender differences between the groups.
Treatment methods were similar to those described by Elewski et al. [1]. Briefly, all patients self-applied 1 drop of efinaconazole 10% solution to the hyponychium and undersurface of the nail every night before bed. The patients in the study group were not restricted with respect to the brand of nail polish. The patients in the study group were also permitted to touch up their polish if needed in the morning without constraint.
The primary outcome, compatibility of topical efinaconazole solution with wearing polish, was assessed at every visit with the following questions: Were you able to apply the medication under your nail? Are you overall satisfied with efinaconazole? Is your polish tacky in the morning? Additionally, patients answered the following question using a Likert or visual analogue scale where 0 represented no alteration in the polish and 10 represented complete destruction of the polish: Is the quality of your polish diminished with use of efinaconazole?
Secondary outcomes included efficacy and safety. Using 3rd-party blinding, DLSO was assessed at baseline and at every subsequent visit using the onychomycosis severity index (OSI), measuring the percent of the target nail involved, and grading the infection from mild to moderate to severe. Nail growth was measured at each visit. Fungal testing was done at screening, 3 months, 7 months, the end of treatment (48 weeks), and the end of the study (52 weeks). Clinical and mycologic cure was evaluated at week 52.
Statistical Analysis
Statistical analysis consisted of 2-tailed t tests to study associations between continuous variables. p values < 0.05 were considered statistically significant.
Results
Thirteen patients in total were enrolled in the study: 7 who identified as frequently wearing toenail polish were placed in the study group, and 6 who agreed to abstain from toenail polish were placed in the control group (Table 1). Eleven patients (6 from the study group and 5 from the control group) completed the 52-week study. Both patients who withdrew did so at week 8 because of personal scheduling difficulties with the study visits. The data were analyzed using the intention-to-treat approach where all randomized patient-recorded data was included in our assessment.
Table 1.
Baseline characteristics of the control and study groups
| Control group (n = 6) | Study group (n = 7) | P value | |
|---|---|---|---|
| Mean toenail thickness, mm | 1.47 | 1.89 | 0.2 |
| Mean OSI score | 12.3 | 11.3 | 0.7 |
| Severe onychomycosis | 33 | 29 | 0.9 |
| Moderate onychomycosis | 67 | 71 | 0.8 |
| Concurrent tinea pedis | 83 | 43 | 0.1 |
Data are presented as percentages, unless otherwise indicated. The p values were obtained with 2-tailed t tests. OSI, onychomycosis severity index.
All patients applied efinaconazole without difficulty, and all but 1 patient from the control group reported satisfaction with efinaconazole. Patients in the study group reported diminishment in polish quality and their polish to be tacky in the morning on average 60% of the time.
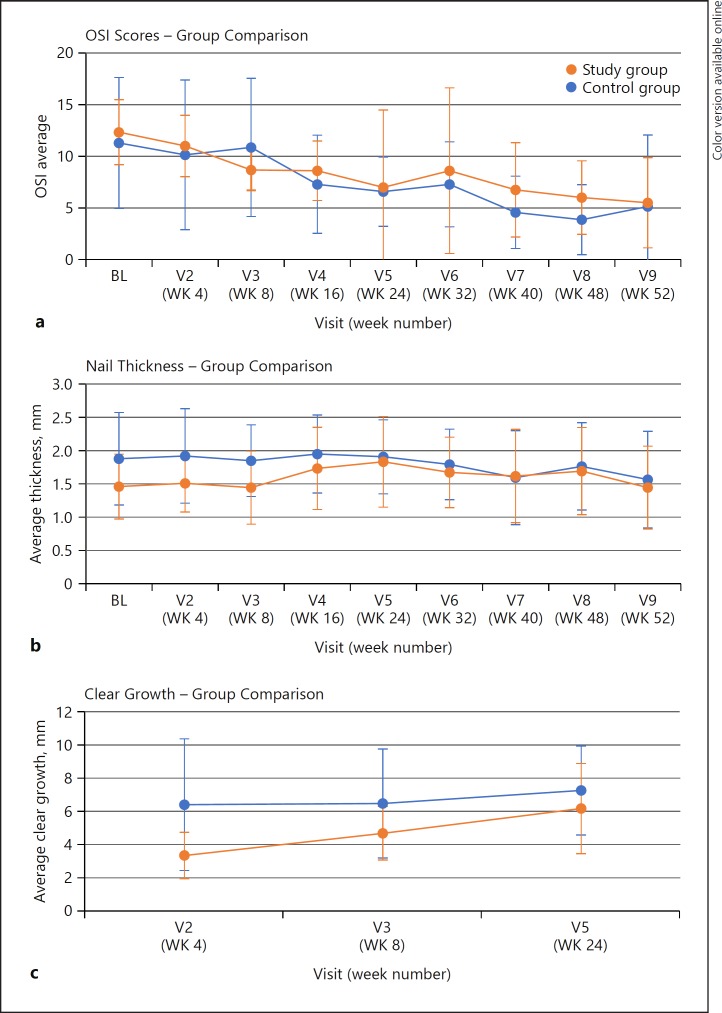
The patients in both groups showed improvement in their OSI scores, nail thickness, and clear nail growth over the course of the study (Fig. 1). Clear growth was measured until week 24; after this time point, clear growth data was not collected on patients who lacked dermatophytomas.
Fig. 1.
Average OSI scores (a), nail thickness (b), and clear nail growth (c). OSI, onychomycosis severity index; BL, baseline; V, visit; WK, week.
Discussion
Our study did not demonstrate a difference in the efficacy of efinaconazole 10% solution in treating onychomycosis (graded by the OSI scoring system) in those applying toenail polish versus those abstaining from toenail polish use, suggesting that toenail polish has no effect on the transungual penetration of the drug. Our results support those of Zeichner et al. [6], who reported that the penetration of efinaconazole was similar in polished and unpolished human thumbnails using a human cadaver nail model.
Although an effective treatment modality in patients wearing toenail polish, efinaconazole can result in an undesirable appearance of the polish, resulting in decreased patient satisfaction and compliance. The patients in our study group reported a disturbance in the toenail polish texture 60% of the time, with 1 patient reporting complete removal of toenail polish subsequent to the application of efinaconazole. Other studies report similar results, noting discoloration of polished nail plates and presence of a tacky texture after just 1 application of efinaconazole [6]. On the other hand, tavaborole 5% solution, an alternative topical treatment for onychomycosis, was not seen to alter the appearance of polished nails [6].
A variety of nail polish brands were used by the study patients, including Essie, Orly, OPI, Mineral Fusion, and Wet & Wild. Top coats and/or base coats included Revlon and China Glaze. Nail plate appearance and color tended to be more stable with OPI, Mineral Fusion, Wet & Wild, and China Glaze, as well as with darker rather than lighter pigments. In addition, greater stability of the nail polish appearance and color was reported in patients using both a top coat and a base coat.
Limitations of this study include the small sample size and range of polish types and brands used by patients in the study group. It is possible that polish quality would have remained stable for longer had all patients used a top and a base coat.
Conclusion
Efinaconazole remains an efficacious treatment modality of DLSO in patients wearing toenail polish; patients need to be made aware of the possibility of alteration in the nail polish appearance and texture prior to initiating treatment. Additional studies are needed to evaluate the appearance of different toenail polish colors and brands during efinaconazole treatment, so that physicians can provide patients with appropriate recommendations. Further research and development is needed to optimize the compatibility of concurrent nail polish use with topical DLSO treatments.
Statement of Ethics
This study was conducted with the approval from the University of Alabama, Birmingham, Institutional Review Board for Human Use. All patients gave their informed consent to participate in this study.
Disclosure Statement
Dr. Elewski has been a consultant and received honorarium payments from the following companies: Celgene, Leo, Lilly, Novartis, Pfizer, Sun, and Valeant. Dr. Elewski has received clinical research support (funds paid to The University of Alabama at Birmingham) from the following companies: Abbvie, Boehringer Ingelheim, Celgene, Incyte, Leo, Lilly, Merck, Novartis, Pfizer, Regeneron, Sun, and Valeant.
Funding Sources
We received a grant from Valeant, and funds went to the University of Alabama, Birmingham.
References
- 1.Elewski BE, Rich P, Pollak R, Pariser DM, Watanabe S, Senda H, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600–608. doi: 10.1016/j.jaad.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Titusville NJ, Sporanox (itraconazole) [package insert] Janssen Pharmaceuticals Inc. 2012 [Google Scholar]
- 3.East Hanover NJ, Lamisil (terbinafine HCl) [package insert] Novartis Pharmaceuticals Corp. 2012 [Google Scholar]
- 4.Vlahovic T, MPharm TM, Chanda S, Zane LT, Coronado D. In vitro nail penetration of tavaborole topical solution, 5%, through nail polish on ex vivo human fingernails. J Drugs Dermatol JDD. 2015;14:675–678. [PubMed] [Google Scholar]
- 5.Vlahovic TC, Coronado D, Chanda S, Merchant T, Zane LT. Evaluation of the appearance of nail polish following daily treatment of ex vivo human fingernails with topical solutions of tavaborole or efinaconazole. J Drugs Dermatol JDD. 2016;15:89–94. [PubMed] [Google Scholar]
- 6.Zeichner JA, Stein Gold L, Korotzer A. Penetration of (14C)-efinaconazole topical solution, 10%, does not appear to be influenced by nail polish. J Clin Aesthet Dermatol. 2014;7:34–36. [PMC free article] [PubMed] [Google Scholar]