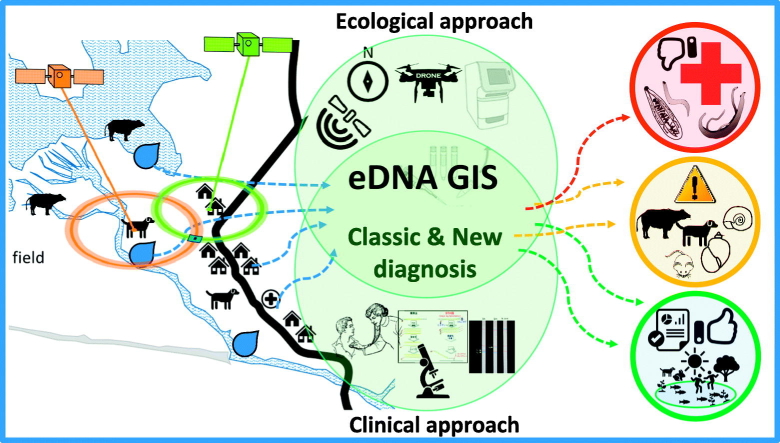
Abstract
The time is passing, and the worms are still a major struggle for local people in Asian countries, especially the less empowered and in a situation of social vulnerability. We are working in the field in Laos, Thailand, and the Philippines where the usual control programs based only on human treatment are partially effective. Areas with mass drug administration could diminish, but not eliminate STHs of endemic areas. The persistence of helminthic NTDs in the environment and animal hosts makes the eradication a very difficult task. Great changes in the landscapes of endemic areas, such as construction of dams, can change the fauna and the lifestyle of local people. Those changes can improve infrastructure, but it can also lead to social vulnerability. The challenge, then, is to conceive new and directed control programs for helminthiasis based on multi- and transdisciplinary approaches diminishing the health gap in a globalized world. In this short review, we summarize the actual scenario concerning the main helminths in Southeast Asia and how an environmental DNA approach and the use of GIS could contribute to surveillance and control programs.
Keywords: Neglected tropical diseases, Worms, Social vulnerability, Environmental DNA, GIS, One-health, Ecohealth
Graphical abstract
1. The persistence of helminths and its consequences
Asia accommodates the world's largest population; in particular, South and Southeast Asia have together more than 2.5 billion people or 1/3 of the world population (United Nations, Department of Economic and Social Affairs, Population Division, 2017). This is an area of ancient human occupation with great human cultural diversity accompanied by a rich diversity of species of plants and animals including parasites (Poulin, 2014; Brown, 2014). This multitude of differences and a warm and humid climate create good conditions for parasites to complete their life cycle readily. This is further facilitated when they are in habitats that are increasingly exposed to human activities such as intensive breeding of domestic animals or encroachment into new areas for various reasons (forced migration, lack of alternatives) due to cultural, social and political issues (Rodrigues et al., 2018; Mitchell et al., 2011; Ejezie and Akpan, 1992). The important helminthes are soil transmitted helminthes (STHs), foodborne helminthes and schistosomes occurring mostly in areas of low-income inhabitants with sub-standard sanitation. Interestingly, people are aware of parasitic disease; however, there is a sentiment that the infection is unavoidable and the treatment uncertain, thus leading to high rates of helminth infection and heavy worm burden (Sato et al., 2018a).
Early reports on helminth infection estimated 1.79 billion people were infected with STHs in 2005 (GBD 2015 Disease and Injury Incidence and Prevalence Collaborators, 2016), 11.2 million people with opisthorchiasis during 2007–2008 (Keiser and Utzinger, 2009), 330 million with schistosomiasis in 2005 (GBD 2015 Disease and Injury Incidence and Prevalence Collaborators, 2016). In recent reports, STHs infection was estimated at 1.65 billion people in 2015 (GBD 2015 Disease and Injury Incidence and Prevalence Collaborators, 2016) and 1.5 billion people in 2018 (WHO (World Health Organization), 2018). For opisthorchiasis, there is no recent updated data, but the prevalence in several areas has been dramatically decreased compared to previous data. Despite specific control measures against the major parasites, the infections persist although at lower rates and lighter worm loads.
In soil-transmitted helminthiasis (STHs), including Strongyloides stercoralis and the zoonotic hookworm Ancylostoma ceylanicum - important agents with high endemicity in Southeast Asia, particularly in Laos (Senephansiri et al., 2017; Sato et al., 2010a; Sato et al., 2011) - heavy infection is not as dangerous as opisthorchiasis and schistosomiasis, but severe morbidity can occur in long-term persisting conditions such as diarrhea, malnutrition, anemia and growth/cognitive stunting, affecting particularly preschool- and school-aged children (Dickson et al., 2000; Gyorkos and Gilbert, 2014). In light and moderate infections, an obvious manifestation is not directly visible but may be collectively conceived by less activity, weakness, decline in working ability, less tolerance to co-infection with other diseases, and increased spending on minor illnesses (WHO (World Health Organization), 2002; Stephenson et al., 2000; Brooker et al., 2004).
The infection by these helminths may not kill the host immediately, but chronic and heavy infection can be lethal, particularly in opisthorchiasis where the infection will lead to cholangiocarcinoma, a fatal disease (Sripa, 2003; Sripa et al., 2011). In addition to opisthorchiasis, schistosomiasis can cause severe morbidity and mortality mediated by a chronic local inflammatory response when eggs become entrapped in host tissues, resulting in intestinal disease, hepatosplenic inflammation, and liver fibrosis and death (Gray et al., 2011). All these morbidity signals can be reflected in the disability-adjusted life years (DALYs), where DALY2015 of STHs is 3.4 million (GBD 2015 DALYs and HALE Collaborators, 2016), DALY2015 of schistosomiasis is 2.6 million (GBD 2015 DALYs and HALE Collaborators, 2016) and DALY2010 of opisthorchiasis is 0.19 million (Havelaar et al., 2015). This is a result of continuous spoiling and is reflected in the difficulty in attention, working and learning which, consequently, lead to a situation of social vulnerability with serious implications in future generations.
2. The anthropocentric view of diseases
Practically, a control of any disease will start with control of morbidity by treatment to reduce worm burden. Mass treatment of STHs reduced heavy infection to moderate and light gradually. Once or twice single dose of benzimidazole per year is the normal practice for STHs diminishing importantly the heavy and moderate infections. Mass treatment with praziquantel on schistosome infections was conducted in Africa and on Fasciola liver fluke infections in South America (Colley et al., 2014; WHO/Department of control of neglected tropical diseases, 2017; Patrick and Isaac-Renton, 1992; Pullan et al., 2014). The second measure taken is building sanitary latrine and the final measure is education on prevention and control of infection. All these actions are emphasized on humans and should be modified according to the local situation. Latrine types need adaptation to different environmental conditions - flooded, desert, mountainous areas, and so on. Latrines are used for trapping parasite eggs from human discharge only but not from animal reservoir hosts. Parasite eggs passed from these animals still contaminate the soils and natural water bodies. Moreover, waste from latrine pits must be treated with care to not reintroduce the eggs to the environment (Nzouebet et al., 2016).
Effective health education needs to be frequently repeated and continuously conducted. At the community level, health education should be carried along with their lifestyle to engage participation and empowerment. At primary schools, learning prevention and control of parasites has been incorporated within school health context. Within the FRESH framework, mass deworming on STHs is conducted together with health education and improving sanitary environment under focused resources (Dhillon and Philip, 1991; World Bank, 2000).
Helminth control approaches usually follow the WHO guidelines. Recommendations for STHs include the use of preventive chemotherapy in risk population groups using albendazole and mebendazole by periodical administration to population at risk; for schistosomiasis the choice drug is praziquantel with the frequency of intervention based on the prevalence of the disease; for foodborne trematodes as O. viverrini, annual MDA is also recommended in high-risk areas and biannual MDA/targeted treatment for people accustomed to eating raw freshwater fish in low-risk areas (WHO (World Health Organization), 2018; WHO/Department of control of neglected tropical diseases, 2017; WHO/Department of control of neglected tropical diseases, 2006; WHO Seventh Meeting of the Working Group on Monitoring of Neglected Tropical Diseases Drug Efficacy, Geneva, 26–27 February 2018, 2018).
In Southeast Asia, 2016 data revealed more than 80 million preschool-aged children were treated through preventive chemotherapy for STHs, equivalent to a regional coverage of 75.4% (WHO/Department of control of neglected tropical diseases, 2017). Due to the logistical difficulties and additional costs of alternative methods to identify and treat infected people, preventive chemotherapy has been the preferred course of action. However, this approach alone is insufficient to break the cycle of infection/reinfection in populations that continue to live in contaminated environments (WHO/Department of control of neglected tropical diseases, 2006).
With the help of WHO to complement the “preventive chemotherapy approach”, several countries in Southeast Asia are currently: developing and implementing multi-sectoral and inter-ministerial collaborations (health, agriculture and rural development) to ensure water safety, adequate sanitation, and monitoring of household animals in the transmission of helminths. These are important steps for helminth transmission control which may be the base for control of STHs (WHO/Department of control of neglected tropical diseases, 2017).
3. Goat and dog roaming patterns and their importance in zoonosis transmission
Rearing goat for subsistence is a common characteristic in Asian families due to easy rearing and the resistance of the species against diseases (Sato et al., 2011; Sato et al., 2014; Mackenzie, 1993). Dogs have an ancient close relationship with humans in several ways with mutual benefit; including companionship, hunting aids, protection, shepherding and many others (Wang et al., 2016). These species are bred in rural and in urban areas and have been demonstrated as important hosts for helminth infection in humans (Sato et al., 2011; Sato et al., 2014; Otake Sato et al., 2017; Chen et al., 2012; El-Shehabi et al., 1999).
3.1. Goats' story
In a rural Village in Lao PDR, “hookworm” prevalence in human fecal samples by Kato-Katz technique was found to be 30.0%. Interestingly, the hookworm eggs were morphologically heterogeneous, and species identification was confirmed by copro-PCR and sequencing. Besides Necator americanus and A. duodenale, common human hookworms, A. caninum, and A. ceylanicum, usually described as animal hookworms, were confirmed to infect the villagers. Moreover, Trichostrongylus spp., another genus of common parasites of domestic animals, was found at a significant infection rate of 21.2% (Sato et al., 2011).
Adult parasites, obtained from hookworm egg-positive human hosts post albendazole treatment, revealed T. colubriformis in 93.5% of analyzed individuals, the same species obtained from goats in the same village, suggesting that T. colubriformis was the main zoonotic species causing “hookworm” infections in the area and causing clinical disease among local residents (Sato et al., 2011; Sato et al., 2014; Watthanakulpanich et al., 2013). The endemicity of this zoonotic trichostrongylid was confirmed in several other places in Lao PDR and Thailand, suggesting the disease is well spread in northeast Thailand and Western Lao PDR region (Phosuk et al., 2013).
It is known that goats have social behavior exhibiting diurnal activity and resting or spending the night in sheltered sites; this behavior is observed in wild goats and is maintained in domesticated goats (Mackenzie, 1993; Shi et al., 2003). The GPS tracking of domestic goats in Lao PDR confirmed the animals had a daily routine of pasturing near houses and returning home in the evening (unpublished data). The infection rate of trichostrongylids in caprines is high, and infective larvae may be spread to all areas close to houses and where vegetables are grown (Sato et al., 2014). The proximity of goats and humans in Laotian communities, where the animals live under the same roof with humans and with the pasture nearby, may facilitate human infection with T. colubriformis, especially by eating raw vegetables (Sato et al., 2011; Sato et al., 2014; Maipanich et al., 2011).
3.2. Dogs run
In rural areas of Asia, dogs are common companion animals owned by local people; they are also bred for guarding and hunting. As dogs usually roam freely outdoors and are exposed to various pathogens including zoonosis agents, it is noted that owners are less concerned about their pets being reservoirs of diseases, and therefore deworming for animals is not a usual practice in such communities (Otake Sato et al., 2017; Chen et al., 2012; El-Shehabi et al., 1999; Aunpromma et al., 2012). These should have been primary conditions for disease control in humans, but this is not the case in many areas.
In our recent study in dogs, we could confirm the presence of more than ten helminth zoonotic species infecting dogs in the Lahanam area (Lao-PDR). Besides the known importance of dogs in the transmission of Ancylostoma spp., Toxocara spp. and Spirometra spp., the roaming pattern of dogs obtained by GPS tracking confirmed it as an important host perpetuating O. viverrini in endemic areas. Dogs routinely accessing water bodies may spread O. viverrini eggs in an environment suitable for fluke development, consequently infecting humans living in the same ecosystem (Otake Sato et al., 2017). This behavior may contribute to further contaminating the environment, thus spreading helminthic infections caused by O. viverrini and other small trematodes and larva migrans, particularly since dogs cohabit with people in the same environment, including all the areas with human activities such as water sources, vegetable farming plots, and homes. This information confirms and reinforces previous studies of environmental contamination where zoonotic parasite eggs were found in areas frequently inhabited by humans with animal access (Maipanich et al., 2011; Chongsuvivatwong et al., 1999; Rai et al., 2000; Steinbaum et al., 2017).
The demographic features of host populations have a direct influence on the transmission and maintenance of infectious diseases (Grenfell and Dobson, 1995; Thrusfield, 2005). Humans have been living closely with several kinds of domesticated/wild animals since ancient times, and the potentially zoonotic helminths should not be overlooked in crafting policies related to infectious disease control.
The study of dog ecology and related anthropological aspects of pet ownership are critical for understanding the epidemiology of canine infectious diseases and also to make decisions in the planning and implementation of dog population management schemes for the control of zoonotic diseases (Bögel et al., 1990; Perry, 1993; Patronek and Rowan, 1995).
Tracking hosts and understanding its behavior in selected areas may give important hints for zoonotic NTDs control programs. Nevertheless, parasitic NTDs control programs in humans should be done in parallel with parasite control in animals within a One-health perspective.
4. Hints in the water: environmental DNA as a tool in eco-epidemiology of helminthic diseases
The relationship among the animal species and the environment is the basic subject of ecology; the composition of an ecosystem is detailed and intricate, and we attempt to understand how each organism fits in a complex network. Therefore, to study specific organisms in the network, we can either look for the organisms themselves or their ecological footprints such as proteins, hormones, cells, and even the DNA they leave behind in the environment (eDNA).
Environmental DNA analysis is a technique to study biota from environmental water or soil samples by detecting DNA from microorganisms and macroorganisms (Ficetola et al., 2008; Minamoto et al., 2012). This method is used for ecological surveys for different types of organisms and is reported in several studies as a useful ecological tool to detect target DNA in the environment, reducing time and cost for surveys (Minamoto et al., 2009; Minamoto et al., 2015; Pitula et al., 2012; Hyman and Collins, 2012; Huver et al., 2015; Hall et al., 2015).
By definition, parasites necessarily need at least one host to survive, so the presence of specific hosts is necessary for the occurrence of any parasite (Lewis et al., 2002). With an eDNA approach, we can determine if a particular habitat contains a parasite or even its host with pinpoint accuracy. Basically, we search for the ecological footprints of target organisms in order to understand their relationship with the environment (Hashizume et al., 2017). This information is extremely important in disease transmission processes because we can work on preventive and educative measures while minimizing human infection.
The diversity of parasites is directly related with the diversity of hosts (Poulin, 2014). However, a recent study showed there is a weak association with host diversity and parasite discovery, mostly because ecological studies are not linked to parasite studies, leading to a lack of valuable information on parasite diversity and impeding the prediction of emerging new diseases (Jorge and Poulin, 2018). Although parasite discovery studies covering huge areas can be problematic because they tend to be laborious and require specialists from sampling to identification, we are attempting to overcome these bottlenecks using eDNA detection in opisthorchiasis and schistosomiasis (Hashizume et al., 2017; Sato et al., 2018b).
It is possible to use an eDNA system for diagnosis in individual samples, which may provide the infection rate in a population. Furthermore, eDNA detection of pathogen and host DNA in environmental samples (i.e. water and soil) can provide additional important information about the pathogens and hosts infection for specific ecotopes (Sato et al., 2018b; Mc Manus et al., 2018). The greatest benefit of eDNA method is collecting data on species distribution from several sites within a short time period because of ease of collecting samples (Hashizume et al., 2017). Another characteristic when using eDNA is the possibility of detection of hosts as well as parasites DNA from a single water sample. Several groups are now targeting eDNA of different groups of organisms (mammals, fishes, mollusks, etc.) (Ficetola et al., 2008; Minamoto et al., 2009; Foote et al., 2012; Thomsen et al., 2012; Takahara et al., 2013; Rees et al., 2014). This method can provide basic information on the local biota, it can be easily applied in the field studies, screening for pathogens and existing hosts, and therefore determine infection risk for determined diseases in any given area.
5. Opisthorchiasis and eDNA in Laos
Opisthorchiasis is a foodborne infection caused by the liver-fluke O. viverrini, it is mainly endemic in Thailand, Lao PDR, Vietnam, and Cambodia (Andrews et al., 2008; Sato et al., 2010b; Sato et al., 2015; Sithithaworn et al., 2012). The lifecycle of O. viverrini is complex, with two intermediate hosts and several mammals including dogs and cats as its final host (Otake Sato et al., 2017; Aunpromma et al., 2012). The first intermediate hosts are freshwater snails of the genus Bithynia, and the second intermediate hosts are cyprinid fish where they encyst and reach the infective stage of metacercaria. Final hosts become infected by ingesting raw or undercooked infected fish (Kaewkes, 2003; Petney et al., 2013).
Eco-epidemiologic studies in opisthorchiasis are done by collecting snail and fish, followed by microscopic confirmation of infection or by shedding tests in snails (Phongsasakulchoti et al., 2005; Kiatsopit et al., 2012). Experimental infections are sometimes necessary for morphological diagnosis (Waikagul, 1998; Rim et al., 2008). These methods are not done on a routine basis and require manpower, physical work, and significant amount of time. On the other hand, studies have been done using real-time PCR assay from intermediate host's DNA to increase the diagnostic sensitivity of O. viverrini (Intapan et al., 2008; Sri-Aroon et al., 2010).
A wide ecoepidemiology study on the habitat and distribution of O. viverrini has not been done yet. Climate changes are triggers for a distribution shift of O. viverrini (Conlan et al., 2011; Utaaker and Robertson, 2015). Also, the transformation of the landscape is occurring at a rapid pace as a consequence of people's migration, irrigation, agricultural expansion, and infrastructure construction (dams, roads, bridges etc.).
In a recent ecoepidemiology study on opisthorchiasis, the occurrence of O. viverrini eDNA and its relationship with the cyprinid fish and snails hosts in an endemic area could be mapped (Hashizume et al., 2017). The greatest advantage of the eDNA method as shown by the study was the compilation of species distribution data from several sites within a short period since sampling can now be done in approximately 5 min per site. It was possible to survey 44 sites with 94 water samples in a total of 6 days in the field with a small team (four people) covering approximately 350 km2. Furthermore, the cost for consumables and reagents needed for DNA concentration, extraction, and real-time PCR is not high (approximately 10 USD per sample).
O. viverrini eDNA was successfully detected in several sites. With the information, it was possible to determine risk areas for consumption of cyprinid fish, which were confirmed afterward microscopically to be infected by O. viverrini metacercariae.
The designing of an eDNA system for the Bithynia snail, intermediate hosts of O. viverrini, is not yet possible. One of the bottlenecks for developing a robust eDNA detection system is the quality of the designed primers; as the database for snails is scarce, there is not enough basic information to develop accurate primers. There is a need for more studies in molecular taxonomy of snails, improving the DNA sequence database of snail species and enabling the design of robust DNA-based snail detection systems.
The eDNA is rapidly degraded in the environment; it is also dependent on its origin, state (intra/extra membranous, particulate, free), and quantity (Barnes and Turner, 2016; Jo et al., 2017; Goldberg et al., 2018). This characteristic makes eDNA detection a good method to detect recently released DNA, demonstrating the actual situation on the sampled site. Another characteristic of eDNA system, as with other DNA based methods, is that it can be sourced from various parts of the parasite or at different developmental stages. In the case of O. viverrini, the sources of eDNA are the different life stages of O. viverrini suspended in the water (egg, miracidium, cercaria), free DNA, or cell fragments.
The eDNA methods had been proven to be efficient to survey and monitor eukaryotic pathogens living in water at specific stages of their lifecycles (Bass et al., 2015). Now, opisthorchiasis ecoepidemiology requires studies on the distribution of bithynid snails (still not possible using eDNA) and cyprinid fishes. The detection of the cyprinid fishes that act as second intermediate hosts of O. viverrini is possible using specific eDNA detection assays and performing metabarcoding (Miya et al., 2015). The use of eDNA is advantageous as it allows understanding of ecological changes in the distribution of O. viverrini and its intermediate hosts; this technique can be used to identify areas with potential disease transmission when only host (definitive, reservoir, and intermediate) eDNA are detected. It is expected that future research using eDNA (for O. viverrini and its hosts) will provide background information (determining risky sites for raw fish consumption, pointing key areas for human treatment) to develop educative strategies for prevention of infection in humans.
Collecting water samples from different sites and analyzing the distribution of the pathogen would be helpful for disease control such as health education to local residents and to make an infection risk map. This eDNA method might be a solution to understand the lifecycle of O. viverrini in a wide-range environment that has not been cleared yet for human activities.
6. Schistosomiasis and eDNA in the Philippines
In the Philippines, schistosomiasis japonica is a long-standing problem affecting mostly the poor living primarily in rural areas (Blas et al., 2004). To date, approximately 12 million Filipinos are living in endemic areas and 2.5 million individuals are directly exposed to infection (Blas et al., 2004; Leonardo et al., 2012; Leonardo et al., 2015; Leonardo et al., 2016). The freshwater snail Oncomelania hupensis quadrasi was discovered by Dr. Marcos Tubangui in 1932 as the intermediate host of Schistosoma japonicum (Leonardo et al., 2016; Blas, 1988–89; Blas, 1991). The snail O. h. quadrasi is present in approximately 3012 bodies of water in the country. Cercariae shed by the snails actively infect mammals, their final hosts. Since some snail colonies may occur in rice fields or in surrounding freshwater bodies supplying water for irrigation, schistosomiasis japonica is considered an occupational hazard as farmers and fishermen are directly exposed and the chance of infection is high (Blas, 1988–89).
Usual epidemiological studies conducted in the Philippines to identify the presence and distribution of S. japonicum involves human and zoonotic infection surveys employing diagnostic procedures through microscopy using stool samples (Kato-Katz) (Leonardo et al., 2008). Recently, serodiagnosis through ELISA and ultrasound of humans were also utilized to confirm cases of schistosomiasis (Leonardo et al., 2015).
Successful control of schistosomiasis requires stringent monitoring and surveillance of active transmission sites, which involves detection of both the Schistosoma parasite and the snail intermediate host such as Oncomelania hupensis. In most cases, this is done via the labor-intensive malacological survey where snails are collected and crushed to check for the presence of the parasite. However, there is a need to complement this method with one that requires less manpower and physical work (Leonardo et al., 2016; Leonardo et al., 2002) that also minimizes exposure of collectors to cercariae in infested freshwater bodies.
6.1. Different view and new approaches to an old disease
Detection of cercarial DNA of S. japonicum from water samples was reported as a promising technique to apply in schistosomiasis-endemic areas in China (Driscoll et al., 2005; Hung and Remais, 2008; Worrell et al., 2011); however, no trials or field tests have yet been done. The detection of eDNA of S. japonicum and the snail intermediate hosts in water samples is a direct indication of transmission. The application of eDNA in field surveys was done in other parasitic species such as Opisthorchis viverrini in some countries in southeast Asia with successful results (Hashizume et al., 2017) and other water-borne pathogens such as Cryptosporidium parvum from stream waters utilizing the small subunit (SSU) rRNA based-PCR-Restriction Fragment Length Polymorphism (RFLP) method (Xiao et al., 2000). Several markers were used for PCR and qPCR assays for S. japonicum detection such as transposons, retrotransposons, (Driscoll et al., 2005; Hung and Remais, 2008) genes expressed during specific life cycle stages (Hung and Remais, 2008; Worrell et al., 2011) and mitochondrial DNA (Kato-Hayashi et al., 2010; Kato-Hayashi et al., 2015). The use of eDNA for S. mansoni was proven to be useful in a study in Madagascar, the use of eDNA detecting active transmission areas could provide information to better focus survey activities on human infection (Sato et al., 2018b). It is a new promising and feasible tool for epidemiology studies on schistosomiasis (Mc Manus et al., 2018).
We have developed and optimized eDNA detection system for S. japonicum and O. h. quadrasi that can be applied on field samples from selected schistosomiasis-endemic areas in the Philippines using qPCR based on the TaqMan System. Successful detection of eDNA through the cox1 gene of S. japonicum and O. h. quadrasi was done both under controlled conditions in the laboratory and in the field (Fornillos et al. submitted). This new technology with a different approach for studies on ecoepidemiology of S. japonicum and O. h. quadrasi in the Philippines can be a new alternative to be utilized in snail and parasite surveillance in endemic areas for public health monitoring.
Complementing the eDNA approach is the utilization of probabilistic methods to identify potential risk areas for humans against exposure to S. japonicum. One such method, developed by Araujo Navas et al. (Araujo Navas et al., 2018), was based on a spatial Bayesian Network (sBN) analysis and used exposure risk factors to humans that include potential snail sites, geographical distribution of snail sites with different snail infection rates, and the cost for communities to access water bodies. They used data on schistosomiasis cases, which include actual household locations, and snail infection prevalence collected from three barangays from Alangalang, Leyte in 2015 and 2016. Additional information from literature was also utilized as prior and conditional probabilities to determine joint probabilities of exposure following the sBN framework. Their results identified areas with high probabilities of exposure to S. japonicum, particularly wet soils in low-sloping areas and those bodies of water that are in close proximity to human habitation.
Data generated from eDNA sampling could therefore potentially supplement probabilistic modelling analyses such as sBN towards a more focused and systematic approach to public health policy, particularly in the implementation of mass drug administration and snail control.
7. Holistic approaches: One-health and Ecohealth
There are several ongoing projects that aim to control helminths in Southeast Asia with limited results (WHO/Department of control of neglected tropical diseases, 2017). There is a need for improvement of multifaceted approaches with multi- and transdisciplinary ways of thinking to make sustainable changes and attain disease elimination/control (WHO/Department of control of neglected tropical diseases, 2017; WHO/Department of control of neglected tropical diseases, 2006). The drugs for treatment are available (WHO Seventh Meeting of the Working Group on Monitoring of Neglected Tropical Diseases Drug Efficacy, Geneva, 26–27 February 2018, 2018), although their effective use is necessary to avoid drug resistance by the parasites. Detecting changes in the environment and its consequences in the occurrence of diseases is a challenge, and the combined use of improved techniques such as GIS and eDNA can be novel approaches to distribution mapping and field surveillance of helminths. Thus, new eDNA systems for different pathogens should be designed and tested using not only water but also soil materials. eDNA can be applied in large scale and aid in control programs effectively and, since only one sampling is enough to detect multiple organisms, important supporting data can be provided for health education and to design effective and sustainable prophylactic and control measures in endemic areas with One-health and Ecohealth thinking.
Acknowledgments
Thanks to Dr. Tippayarat Yoonuan for the critical reading of the manuscript. Thanks to Dr. Surapol Sanguankiat and Mr. Nirandon Homsuwan for their generous assistance in our field surveys. This study was partially supported by: Department of Science and Technology-Philippine Council for Industry, Energy, and Emerging Technology Research and Development (DOST-PCIEERD) and the Natural Sciences Research Institute, University of the Philippines.
References
- Andrews R.H., Sithithaworn P., Petney T.N. Opisthorchis viverrini: an underestimated parasite in world health. Trends Parasitol. 2008;24:497–501. doi: 10.1016/j.pt.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo Navas A.L., Soares Magalhaes R.J., Osei F., Fornillos R.J.C., Leonardo L.R., Stein A. Modelling local areas of exposure to Schistosoma japonicum in a limited survey data environment. Parasit. Vectors. 2018;11:465. doi: 10.1186/s13071-018-3039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aunpromma S., Tangkawattana P., Papirom P., Kanjampa P., Tesana S., Sripa B., Tangkawattana S. High prevalence of Opisthorchis viverrini infection in reservoir hosts in four districts of Khon Kaen Province, an opisthorchiasis endemic area of Thailand. Parasitol. Int. 2012;61:60–64. doi: 10.1016/j.parint.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Barnes M.A., Turner C.R. The ecology of environmental DNA and implications for conservation genetics. Conserv. Genet. 2016;17:1. [Google Scholar]
- Bass D., Stentiford G.D., Littlewood D.T.J., Hartikainen H. Diverse applications of environmental DNA methods in parasitology. Trends Parasitol. 2015;31(10):499–513. doi: 10.1016/j.pt.2015.06.013. [DOI] [PubMed] [Google Scholar]
- Blas B.L. DOH Schistosomiasis Control Service; 1988–89. Handbook for the Control of Schistosomiasis Japonica: Part II Guide to Control Operations; pp. 22–24. [Google Scholar]
- Blas B. Monograph on Schistosoma japonicum Infection in the Philippines. Schistosomiasis Control Service, Department of Health; 1991. Handbook for the control of schistosomiasis japonica; pp. 10–13. [Google Scholar]
- Blas B.L., Rosales M.I., Lipayon I.L., Yasuraoka K., Matsuda H., Hayashi M. The schistosomiasis problem in the Philippines: a review. Parasitol. Int. 2004;53(2):127–134. doi: 10.1016/j.parint.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Bögel K., Frucht K., Drysdale G., Remfry J., World Health Organization, Veterinary Public Health Unit . World Health Organization; Geneva: 1990. Guidelines for Dog Population Management; p. 116.http://www.who.int/iris/handle/10665/61417 [Google Scholar]
- Brooker S., Bethony J., Hotez P.J. Human hookworm infection in the 21st century. Adv. Parasitol. 2004;58:197–288. doi: 10.1016/S0065-308X(04)58004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.H. Why are there so many species in the tropics? J. Biogeogr. 2014;41:8–22. doi: 10.1111/jbi.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Xu M.J., Zhou D.H., Song H.Q., Wang C.R., Zhu X.Q. Canine and feline parasitic zoonoses in China. Parasit. Vectors. 2012;5:152. doi: 10.1186/1756-3305-5-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chongsuvivatwong V., Uga S., Nagnaen W. Soil contamination and infections by soil-transmitted helminths in an endemic village in southern Thailand. Southeast Asian J. Trop. Med. Public Health. 1999;30(1):64–67. [PubMed] [Google Scholar]
- Colley D.G., Bustinduy A.L., Secor W.E., King C.H. Human schistosomiasis. Lancet. 2014;383(9936):2253–2264. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlan J.V., Sripa B., Attwood S., Newton P.N. A review of parasitic zoonoses in a changing Southeast Asia. Vet. Parasitol. 2011;182:22–40. doi: 10.1016/j.vetpar.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Dhillon H.S., Philip L. World Health Organization, Division of Health Education & International Conference on Health Promotion, Supportive Environments for Health. World Health Organization Geneva; 1991. Health promotion in developing countries: briefing book to the Sundsvall Conference on Supportive Environments, Sundsvall, Sweden.http://www.who.int/iris/handle/10665/61377 [Google Scholar]
- Dickson R., Awasthi S., Demellweek C., Williamson P. Anthelmintic drugs for treating worms in children: effects on growth and cognitive performance. Cochrane Database Syst. Rev. 2000;2 doi: 10.1002/14651858.CD000371. [DOI] [PubMed] [Google Scholar]
- Driscoll A.J., Kyle J.L., Remais J. Development of a novel PCR assay capable of detecting a single Schistosoma japonicum cercaria recovered from Oncomelania hupensis. Parasitology. 2005;131(4):497–500. doi: 10.1017/S0031182005007961. [DOI] [PubMed] [Google Scholar]
- Ejezie G.C., Akpan I.F. Human ecology and parasitic infections. 1. The effect of occupation on the prevalence of parasitic infections in Calabar, Nigeria. J. Hyg. Epidemiol. Microbiol. Immunol. 1992;36(2):161–167. [PubMed] [Google Scholar]
- El-Shehabi F.S., Abdef-Afez S.K., Kamhawi S.A. Prevalence of intestinal helminths of dogs and fox from Jordan. Parasitol. Res. 1999;85:928–934. doi: 10.1007/s004360050660. [DOI] [PubMed] [Google Scholar]
- Ficetola G.F., Miaud C., Pompanon F., Taberlet P. Species detection using environmental DNA from water samples. Biol. Lett. 2008;4(4):423–425. doi: 10.1098/rsbl.2008.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote A.D., Thomsen P.F., Sveegaard S., Wahlberg M., Kielgast J., Kyhn L.A. Investigating the potential use of environmental DNA (eDNA) for genetic monitoring of marine mammals. PLoS One. 2012;7 doi: 10.1371/journal.pone.0041781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2015 DALYs and HALE Collaborators Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1603–1658. doi: 10.1016/S0140-6736(16)31460-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg C.S., Strickler K.M., Fremier A.K. Degradation and dispersion limit environmental DNA detection of rare amphibians in wetlands: increasing efficacy of sampling designs. Sci. Total Environ. 2018;633:695–703. doi: 10.1016/j.scitotenv.2018.02.295. [DOI] [PubMed] [Google Scholar]
- Gray D.J., Ross A.G., Li Y.S., McManus D.P. Diagnosis and management of schistosomiasis. BMJ. 2011;342:d2651. doi: 10.1136/bmj.d2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenfell B., Dobson A. Cambridge University Press A; Cambridge: 1995. Ecology of Infectious Diseases in Natural Populations (Publications of the Newton Institute) [Google Scholar]
- Gyorkos T.W., Gilbert N.L. Blood drain: soil-transmitted helminths and anemia in pregnant women. PLoS Negl. Trop. Dis. 2014;8(7) doi: 10.1371/journal.pntd.0002912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall E.M., Crespi E.J., Goldberg C., Brunner J.L. Evaluating environmental DNA-based quantification of ranavirus infection in wood frog populations. Mol. Ecol. Resour. 2015;16:423–433. doi: 10.1111/1755-0998.12461. [DOI] [PubMed] [Google Scholar]
- Hashizume H., Sato M., Sato M.O., Ikeda S., Yoonuan T., Sanguankiat S. Application of environmental DNA analysis for the detection of Opisthorchis viverrini DNA in water samples. Acta Trop. 2017;169:1–7. doi: 10.1016/j.actatropica.2017.01.008. [DOI] [PubMed] [Google Scholar]
- Havelaar A.H., Kirk M.D., Torgerson P.R., Gibb H.J., Hald T., Lake R.J., World Health Organization Foodborne Disease Burden Epidemiology Reference Group World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015;12(12) doi: 10.1371/journal.pmed.1001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung Y.W., Remais J. Quantitative detection of Schistosoma japonicum cercariae in water by real-time PCR. PLoS Negl. Trop. Dis. 2008;2(11) doi: 10.1371/journal.pntd.0000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huver J.R., Koprivnikar J., Johnson P.T.J., Whyard S. Development and application of an eDNA method to detect and quantify a pathogenic parasite in aquatic ecosystems. Ecol. Appl. 2015;25:991–1002. doi: 10.1890/14-1530.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman O.J., Collins J.P. Evaluation of a filtration-based method for detecting Batrachochytrium dendrobatidis in natural bodies of water. Dis. Aquat. Org. 2012;97:185–195. doi: 10.3354/dao02423. [DOI] [PubMed] [Google Scholar]
- Intapan P.M., Thanchomnang T., Lulitanond V., Phongsaskulchoti P., Maleewong W. Detection of Opisthorchis viverrini in infected bithynid snails by real-time fluorescence resonance energy transfer PCR-based method and melting curve analysis. Parasitol. Res. 2008;103:649–655. doi: 10.1007/s00436-008-1026-0. [DOI] [PubMed] [Google Scholar]
- Jo T., Murakami H., Masuda R., Sakata M.K., Yamamoto S., Minamoto T. Rapid degradation of longer DNA fragments enables the improved estimation of distribution and biomass using environmental DNA. Mol. Ecol. Resour. 2017;17(6):e25–e33. doi: 10.1111/1755-0998.12685. [DOI] [PubMed] [Google Scholar]
- Jorge F., Poulin R. Poor geographical match between the distributions of host diversity and parasite discovery effort. Proc. R. Soc. B. 2018;285:20180072. doi: 10.1098/rspb.2018.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaewkes S. Taxonomy and biology of liver flukes. Acta Trop. 2003;88:177–186. doi: 10.1016/j.actatropica.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Kato-Hayashi N., Kirinoki M., Iwamura Y., Kanazawa T., Kitikoon V., Matsuda H., Chigusa Y. Identification and differentiation of human schistosomes by polymerase chain reaction. Exp. Parasitol. 2010;124(3):325–329. doi: 10.1016/j.exppara.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Kato-Hayashi N., Leonardo L.R., Arevalo N.L., Tagum M.N., Apin J., Agsolid L.M. Detection of active schistosome infection by cell-free circulating DNA of Schistosoma japonicum in highly endemic areas in Sorsogon Province, the Philippines. Acta Trop. 2015;141(Pt B):178–183. doi: 10.1016/j.actatropica.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Keiser J., Utzinger J. Food-borne trematodiases. Clin. Microbiol. Rev. 2009;22(3):466–483. doi: 10.1128/CMR.00012-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiatsopit N., Sithithaworn P., Saijuntha W., Boonmars T., Tesana S., Sithithaworn J. Exceptionally high prevalence of infection of Bithynia siamensis goniomphalos with Opisthorchis viverrini cercariae indifferent wetlands in Thailand and Lao PDR. Am. J. Trop. Med. Hyg. 2012;86:464–469. doi: 10.4269/ajtmh.2012.11-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardo L.R., Acosta L.P., Olveda R.M., Aligui G.D.L. Difficulties and strategies in the control of schistosomiasis in the Philippines. Acta Trop. 2002;82(2):295–299. doi: 10.1016/s0001-706x(02)00022-0. [DOI] [PubMed] [Google Scholar]
- Leonardo L.R., Rivera P., Saniel O., Villacorte E., Crisostomo B., Hernandez L. Prevalence survey of schistosomiasis in Mindanao and the Visayas, The Philippines. Parasitol. Int. 2008;57(3):246–251. doi: 10.1016/j.parint.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Leonardo L., Rivera P., Saniel O., Villacorte E., Lebanan M.A., Crisostomo B. A national baseline prevalence survey of schistosomiasis in the Philippines using stratified two-step systematic cluster sampling design. J. Trop. Med. 2012;936128:1–8. doi: 10.1155/2012/936128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardo L., Rivera P., Saniel O., Solon J.A., Chigusa Y., Villacorte E. New endemic foci of schistosomiasis infections in the Philippines. Acta Trop. 2015;141:354–360. doi: 10.1016/j.actatropica.2013.03.015. [DOI] [PubMed] [Google Scholar]
- Leonardo L., Chigusa Y., Kikuchi M., Kato-Hayashi N., Kawazu S., Angeles J.M. Schistosomiasis in the Philippines: challenges and some successes in control. Southeast Asian J. Trop. Med. Public Health. 2016;47(4):651–666. [Google Scholar]
- Lewis E.E., Campbell J.F., Sukhdeo M.V.K., editors. The Behavioural Ecology of Parasites. CABI; 2002. (384 pp) [Google Scholar]
- Mackenzie D. In: Goat Husbandry. 5th ed. Goodwin R., editor. Faber and Faber; 1993. p. 334. [Google Scholar]
- Maipanich W., Chaisiri K., Yoonuan T., Sato M., Sato M.O., Pongvongsa T. Zoonotic helminth contamination of the environment in rural villages of southern Lao PDR. J. Trop. Med. Parasitol. 2011;34:54–61. [Google Scholar]
- Mc Manus D.P., Gordon C., Weerakoon K.G.A.D. Testing of water samples for environmental DNA as a surveillance tool to assess risk of schistosome infection in a locality. Int. J. Infect. Dis. 2018 doi: 10.1016/j.ijid.2018.09.022. [DOI] [PubMed] [Google Scholar]
- Minamoto T., Honjo M.N., Uchii K., Yamanaka H., Suzuki A.A., Kohmatsu Y. Detection of cyprinid herpesvirus 3 DNA in river water during and after an outbreak. Vet. Microbiol. 2009;135(3–4):261–266. doi: 10.1016/j.vetmic.2008.09.081. [DOI] [PubMed] [Google Scholar]
- Minamoto T., Yamanaka H., Takahara T., Honjo M.N., Kawabata Z. Surveillance of fish species composition using environmental DNA. Limnology. 2012;13(2):193–197. [Google Scholar]
- Minamoto T., Pu X., Xie J., Dong Y., Wu D., Kong H. Monitoring of fish pathogenic viruses in natural lakes in Yunnan, China. Limnology. 2015;16:69–77. [Google Scholar]
- Mitchell P.D., Anastasiou E., Syon D. Human intestinal parasites in crusader Acre: evidence for migration with disease in the medieval period. Int. J. Paleopathol. 2011;1(3–4):132–137. doi: 10.1016/j.ijpp.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Miya M., Sato Y., Fukunaga T., Sado T., Poulsen J.Y., Sato K. Mifish, a set of universal PCR primers for metabarcoding environmental DNA from fishes: detection of more than 230 subtropical marine species. R. Soc. Open Sci. 2015;2:150088. doi: 10.1098/rsos.150088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nzouebet W.A.L., Noumsi I.M.K., Rechenburg A. Prevalence and diversity of intestinal helminth eggs in pit latrine sludge of a tropical urban area. J. Water Sanit. Hyg. Dev. 2016;6(4):622–630. [Google Scholar]
- Otake Sato M., Sato M., Yoonuan T., Pongvongsa T., Sanguankiat S., Kounnavong S. The role of domestic dogs in the transmission of zoonotic helminthes in a rural area of Mekong river basin. Acta Parasitol. 2017;62(2):393–400. doi: 10.1515/ap-2017-0047. [DOI] [PubMed] [Google Scholar]
- Patrick D.M., Isaac-Renton J. Praziquantel failure in the treatment of Fasciola hepatica. Can. J. Infect. Dis. 1992;3(1):33–36. doi: 10.1155/1992/864093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patronek G.J., Rowan A.N. Determining dog and cat numbers and population dynamics. Anthrozoös. 1995;8(4):199–205. [Google Scholar]
- Perry B.D. Dog ecology in eastern and southern Africa: implications for rabies control. Onderstepoort J. Vet. Res. 1993;60:429–436. [PubMed] [Google Scholar]
- Petney T.N., Andrews R.N., Saijuntha W., Wenz-Mücke A., Sithithaworn P. The zoonotic, fish-borne liver flukes Clonorchis sinensis, Opisthorchis felineus and Opisthorchis viverrini. Int. J. Parasitol. 2013;43:1031–1046. doi: 10.1016/j.ijpara.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Phongsasakulchoti P., Sri-Aroon P., Kerdpuech Y. Emergence of Opisthorchis viverrini cercariae from naturally infected Bithynia (Digoniostoma) siamensis goniomphalos. Southeast Asian J. Trop. Med. Public Health. 2005;36:189–191. [PubMed] [Google Scholar]
- Phosuk I., Intapan P.M., Sanpool O., Janwan P., Thanchomnang T., Sawanyawisuth K. Molecular evidence of Trichostrongylus colubriformis and Trichostrongylus axei infections in humans from Thailand and Lao PDR. Am. J. Trop. Med. Hyg. 2013;89(2):376–379. doi: 10.4269/ajtmh.13-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitula J.S., Dyson W.D., Bakht H.B., Njoku I., Chen F. Temporal distribution of genetically homogenous ‘free-living’ Hematodinium sp. in a Delmarva coastal ecosystem. Aquat. Biosyst. 2012;8(16) doi: 10.1186/2046-9063-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin R. Parasite biodiversity revisited: frontiers and constraints. Int. J. Parasitol. 2014;44:581–589. doi: 10.1016/j.ijpara.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Pullan R.L., Smith J.L., Jasrasaria R., Brooker S.J. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit. Vectors. 2014;7:37. doi: 10.1186/1756-3305-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai S.K., Uga S., Ono K., Rai G., Matsumura T. Contamination of soil with helminth parasite eggs in Nepal. Southeast Asian J. Trop. Med. Public Health. 2000;31(2):388–393. [PubMed] [Google Scholar]
- Rees H.C., Maddison B.C., Middleditch D.J., Patmore J.R.M., Gough K.C. The detection of aquatic animal species using environmental DNA – a review of eDNA as a survey tool in ecology. J. Appl. Ecol. 2014;51:1450–1459. [Google Scholar]
- Rim H.J., Sohn W.M., Yong T.S., Eom K.S., Chai J.Y., Min D.Y. Fishborne trematode metacercariae detected in freshwater fish from Vientiane municipality and Savannakhet province, Lao PDR. Korean J. Parasitol. 2008;46:253–260. doi: 10.3347/kjp.2008.46.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues P.T., Valdivia H.O., de Oliveira T.C., Alves J.M.P., Duarte A.M.R.C., Cerutti-Junior C. Human migration and the spread of malaria parasites to the New World. Sci. Rep. 2018;8(1):1993. doi: 10.1038/s41598-018-19554-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Sanguankiat S., Yoonuan T., Pongvongsa T., Keomoungkhoun M., Phimmayoi I., Boupa B., Moji K., Waikagul J. Copro-molecular identification of infections with hookworm eggs in rural Lao PDR. Trans. R. Soc. Trop. Med. Hyg. 2010;104(9):617–622. doi: 10.1016/j.trstmh.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Sato M., Pongvongsa T., Sanguankiat S., Yoonuan T., Dekumyoy P., Kalambaheti T. Copro-DNA diagnosis of Opisthorchis viverrini and Haplorchis taichui infection in an endemic area of Lao PDR. Southeast Asian J. Trop. Med. Public Health. 2010;41:28–35. [PubMed] [Google Scholar]
- Sato M., Yoonuan T., Sanguankiat S., Nuamtanong S., Pongvongsa T., Phimmayoi I. Short report: human Trichostrongylus colubriformis infection in a rural village in Laos. Am. J. Trop. Med. Hyg. 2011;84(1):52–54. doi: 10.4269/ajtmh.2011.10-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M.O., Sato M., Chaisiri K., Maipanich W., Yoonuan T., Sanguankiat S. Nematode infection among ruminants in monsoon climate (Ban-Lahanam, Lao PDR) and its role as food-borne zoonosis. Rev. Bras. Parasitol. Vet. 2014;23(1):80–84. doi: 10.1590/s1984-29612014011. [DOI] [PubMed] [Google Scholar]
- Sato M., Pongvongsa T., Sanguankiat S., Yoonuan T., Kobayashi J., Boupha B. Patterns of trematode infections of Opisthorchis viverrini (Opisthorchiidae) and Haplorchis taichui (Heterophyidae) in human populations from two villages in Savannakhet Province, Lao PDR. J. Helminthol. 2015;89:439–445. doi: 10.1017/S0022149X14000261. [DOI] [PubMed] [Google Scholar]
- Sato M.O., Sato M., Yanagida T., Waikagul J., Pongvongsa T., Sako Y. Taenia solium, Taenia saginata, Taenia asiatica, their hybrids and other helminthic infections occurring in a neglected tropical diseases' highly endemic area in Lao PDR. PLoS Negl. Trop. Dis. 2018;12(2) doi: 10.1371/journal.pntd.0006260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M.O., Rafalimanantsoa A., Ramarokoto C., Rahetilahy A.M., Ravoniarimbinina P., Kawai S. Usefulness of environmental DNA for detecting Schistosoma mansoni occurrence sites in Madagascar. Int. J. Infect. Dis. 2018 doi: 10.1016/j.ijid.2018.08.018. [DOI] [PubMed] [Google Scholar]
- Senephansiri P., Laummaunwai P., Laymanivong S., Boonmar T. Status and risk factors of Strongyloides stercoralis infection in rural communities of Xayaburi province, Lao PDR. Korean J. Parasitol. 2017;55(5):569–573. doi: 10.3347/kjp.2017.55.5.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Dunbar R.I.M., Buckland D., Miller D. Daytime activity budgets of feral goats (Capra hircus) on the Isle of Rum: influence of season, age, and sex. Can. J. Zool. 2003;81:803–815. [Google Scholar]
- Sithithaworn P., Andrews R.H., Nguyen V.D., Wongsaroj T., Sinuon M., Odermatt P. The current status of opisthorchiasis in the Mekong Basin. Parasitol. Int. 2012;61:10–16. doi: 10.1016/j.parint.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sri-Aroon P., Chusongsang P., Chusongsang Y., Surinthwong P., Butraporn P., Lohachit C. Snails and trematode infection after Indian Ocean tsunami in Phang-Nga province, Southern Thailand. Southeast Asian J. Trop. Med. Public Health. 2010;41:48–60. [PubMed] [Google Scholar]
- Sripa B. Pathobiology of opisthorchiasis: an update. Acta Trop. 2003;88:209–220. doi: 10.1016/j.actatropica.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Sripa B., Bethony J.M., Sithithaworn P., Kaewkes S., Mairiang E., Loukas A. Opisthorchiasis and Opisthorchis-associated cholangiocarcinoma in Thailand and Laos. Acta Trop. 2011;120(S1):158–168. doi: 10.1016/j.actatropica.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbaum L., Kwong L.H., Ercumen A., Negash M.S., Lovely A.J., Njenga S.M. Detecting and enumerating soil-transmitted helminth eggs in soil: new method development and results from field testing in Kenya and Bangladesh. PLoS Negl. Trop. Dis. 2017;11(4) doi: 10.1371/journal.pntd.0005522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson L.S., Latham M.C., Ottesen E.A. Malnutrition and parasitic helminth infections. Parasitology. 2000;121(Suppl):23–28. doi: 10.1017/s0031182000006491. [DOI] [PubMed] [Google Scholar]
- Takahara T., Minamoto T., Doi H. Using environmental DNA to estimate the distribution of an invasive fish species in ponds. PLoS One. 2013;8 doi: 10.1371/journal.pone.0056584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen P.F., Kielgast J.O.S., Iversen L.L., Wiuf C., Rasmussen M., Gilbert M.T.P. Monitoring endangered freshwater biodiversity using environmental DNA. Mol. Ecol. 2012;21:2565–2573. doi: 10.1111/j.1365-294X.2011.05418.x. [DOI] [PubMed] [Google Scholar]
- Thrusfield M. Surveys. In: Thrusfield M., editor. Veterinary Epidemiology. Blackwell; Oxford: 2005. pp. 228–242. [Google Scholar]
- United Nations, Department of Economic and Social Affairs, Population Division . 2017. World Population Prospects: The 2017 Revision, Key Findings and Advance Tables. (Working Paper No. ESA/P/WP/248) [Google Scholar]
- Utaaker K.S., Robertson L.J. Climate change and foodborne transmission of parasites: a consideration of possible interactions and impacts for selected parasites. Food Res. Int. 2015;68:16–23. [Google Scholar]
- Waikagul J. Opisthorchis viverrini metacercaria in Thai freshwater fish. Southeast Asian J. Trop. Med. Public Health. 1998;29:324–326. [PubMed] [Google Scholar]
- Wang G.D., Zhai W., Yang H.C., Wang L., Zhong L., Liu Y.H. Out of southern East Asia: the natural history of domestic dogs across the world. Cell Res. 2016;26:21–33. doi: 10.1038/cr.2015.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watthanakulpanich D., Pongvongsa T., Sanguankiat S., Nuamtanong S., Maipanich W., Yoonuan T. Prevalence and clinical aspects of human Trichostrongylus colubriformis infection in Lao PDR. Acta Trop. 2013;126(1):37–42. doi: 10.1016/j.actatropica.2013.01.002. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization) WHO Technical Series Report 912. 2002. Prevention and control of schistosomiasis and soil-transmitted helminthiasis, Geneva. [PubMed] [Google Scholar]
- WHO (World Health Organization) Soil-transmitted helminth infections. 2018. http://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections
- WHO Seventh Meeting of the Working Group on Monitoring of Neglected Tropical Diseases Drug Efficacy, Geneva, 26–27 February 2018. World Health Organization; Geneva: 2018. [Google Scholar]
- WHO/Department of control of neglected tropical diseases . In: Preventive Chemotherapy in Human Helminthiasis – Coordinated Use of Anthelminthic Drugs in Control Interventions. A Manual for Health Professionals and Programme Managers. Dirk Engels/Preventive Chemotherapy, editor. WHO Press; Geneve: 2006. (61pp) [Google Scholar]
- WHO/Department of control of neglected tropical diseases Schistosomiasis and soil-transmitted helminthiases: number of people treated in 2016. Wkly Epidemiol. Rec. 2017;49(92):749–760. [PubMed] [Google Scholar]
- World Bank . Focusing Resources on Effective School Health (FRESH) Series, Washington. 2000. Focusing resources on effective school health: a FRESH start to enhancing the quality and equity of education (English) [Google Scholar]
- Worrell C., Xiao N., Vidal J.E., Chen L., Zhong B., Remais J. Field detection of Schistosoma japonicum cercariae in environmental water samples by quantitative PCR. Appl. Environ. Microbiol. 2011;77(6):2192–2195. doi: 10.1128/AEM.01561-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L., Alderisio K., Limor J., Royer M., Lal A.A. Identification of species and sources of Cryptosporidium oocysts in storm waters with a small-subunit rRNA-based diagnostic and genotyping tool. Appl. Environ. Microbiol. 2000;66(12):5492–5498. doi: 10.1128/aem.66.12.5492-5498.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]