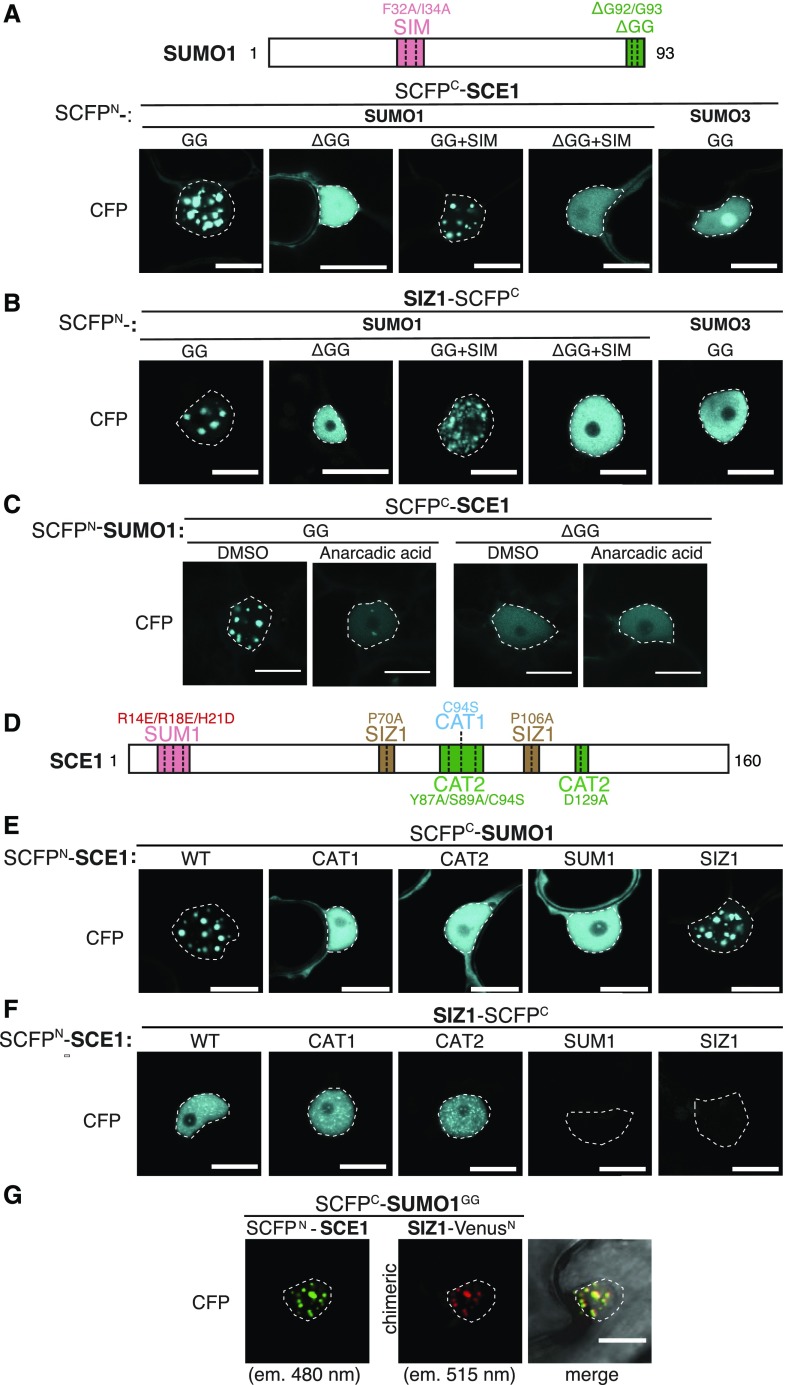
Figure 1.
SUMO nuclear body formation depends on SCE1 conjugation activity. A, Nuclear localization pattern of SUMO1∙SCE1 BiFC pairs, including mutant variants of SUMO1 and the SUMO3∙SCE1 BiFC pair. Included above is a schematic representation of residues mutated and/or deleted in SUMO1 (GG, mature SUMO; SIM, F32A+I34A; ΔGG, deletion of the C-terminal diGly motif). B, Similar to A; nuclear localization pattern of SUMO1/3-SIZ1 BiFC pairs including mutant variants of SUMO1. C, Nuclear localization pattern of the SUMO1∙SCE1 complex in response to anacardic acid inhibition of SUMO conjugation (100 μm in 1% [v/v] DMSO) 1.5 h postinfiltration. D, Schematic representation of SCE1 mutants and their substitutions. CAT1, catalytic Cys residue mutated; CAT2, binding pocket for the ψKxE SUMO acceptor motif mutated; SUM1, noncovalent association of SUMO disrupted; SIZ1, SIZ1-binding disrupted. E, Nuclear localization pattern of SUMO1∙SCE1 BiFC pairs, including mutant variants of SCE1: SCE1CAT1, SCE1CAT2, SCE1SUM1, and SCE1SIZ1. F, Nuclear localization pattern of SCE1∙SIZ1 BiFC pairs, including mutant variants of SCE1: SCE1CAT1, SCE1CAT2, SCE1SUM1, and SCE1SIZ1. G, Multicolor BiFC of SUMO1GG, SCE1, and SIZ1 showing nuclear localization pattern. The micrographs show the nuclear signal of the reconstituted SCFPN/SCFPC (SCE1∙SUMO1GG) and VenusN/SCFPC (SIZ∙SUMO1GG) fluorophores and their merged signals. The two chimeric BiFC combinations differ in their excitation and emission spectra. The BiFC pairs in A to F were fused to two halves of SCFP (SCFPN + SCFPC) with the orientation of the fusions indicated. Scale bars, 20 µm. All micrographs were taken in N. benthamiana epidermal leaf cells 2 to 3 d post-agro-infiltration with strains expressing the indicated constructs; nuclei are outlined with white lines. Supplemental Figure S3 depicts for A to F an overlay of the DIC and CFP images of the nuclei shown and a zoom-out (A′–F′) depicting the BiFC signal in the entire cell.