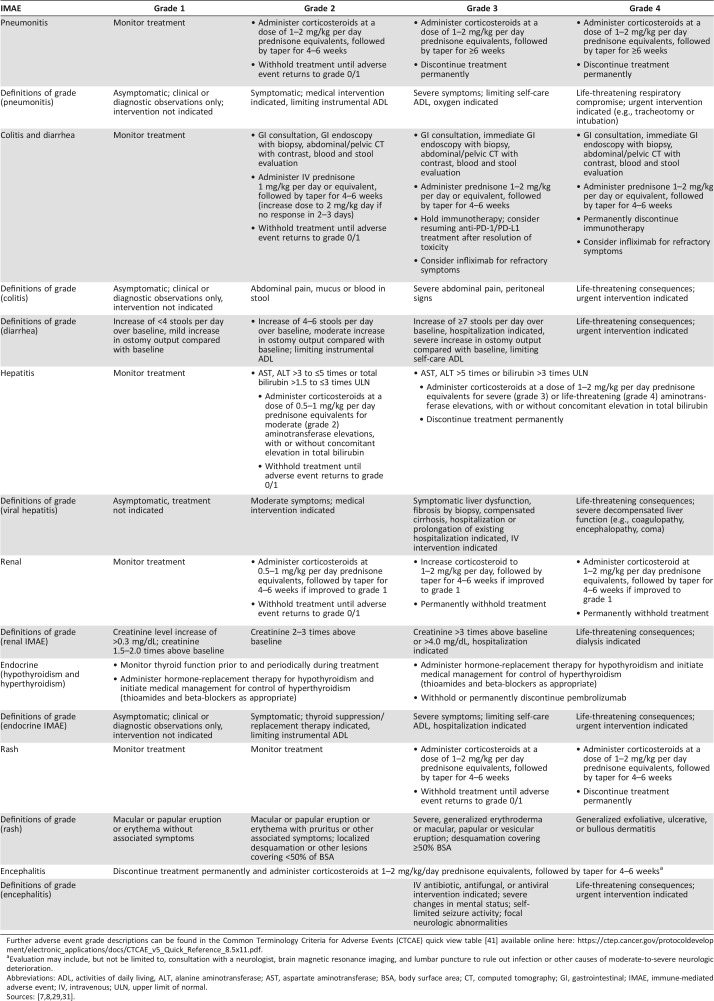
Table 2. Management of IMAEs, by gradea and organ system.
Further adverse event grade descriptions can be found in the Common Terminology Criteria for Adverse Events (CTCAE) quick view table [41] available online here: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf.
Evaluation may include, but not be limited to, consultation with a neurologist, brain magnetic resonance imaging, and lumbar puncture to rule out infection or other causes of moderate‐to‐severe neurologic deterioration.
Abbreviations: ADL, activities of daily living, ALT, alanine aminotransferase; AST, aspartate aminotransferase; BSA, body surface area; CT, computed tomography; GI, gastrointestinal; IMAE, immune‐mediated adverse event; IV, intravenous; ULN, upper limit of normal.