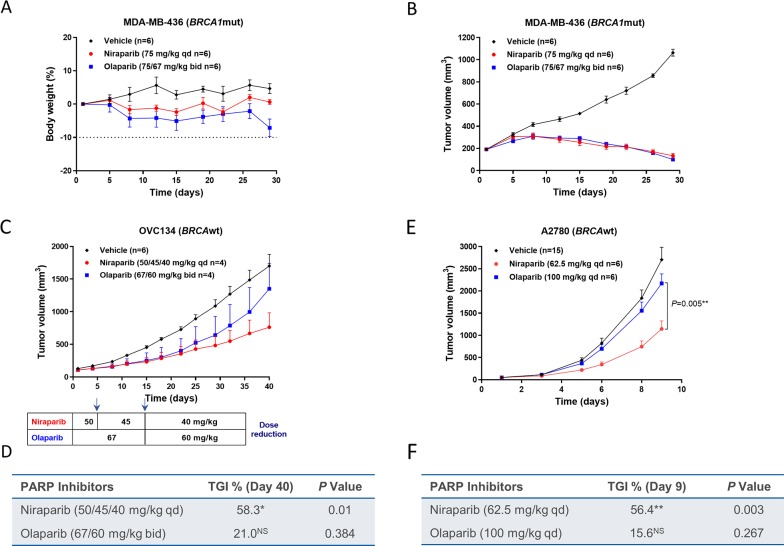
Figure 3.
Effect of niraparib and olaparib on tumor volume and body weight in a BRCA1mut MDA-MB-436 TNBC cell line-derived xenograft model (A & B), BRCAwt OVC134 ovarian cancer PDX model (C & D), and BRCAwt A2780 ovarian cancer cell line-derived xenograft model (E & F). (A) Percentage change in body weight and (B) tumor volume of the MDA-MB-436 model treated with niraparib or olaparib at the maximum tolerated dose. Niraparib was administered at 75 mg/kg daily for 28 days. Olaparib was administered at 75 mg/kg twice daily for 7 days and then at 67 mg/kg twice daily for 21 days due to significant body weight loss observed in the olaparib-treated group. (C) Tumor growth of the OVC134 model treated with niraparib or olaparib at the maximum tolerated dose. Dose reductions due to body weight loss are indicated in the chart below the growth curve for both the niraparib- and olaparib-treated groups. Two mice from each treated group were given extensive dose holidays and therefore were excluded from final analysis (D) Table summarizing TGI and P value calculated by Student’s t test for niraparib or olaparib compared to vehicle on day 40, *P<0.05, NS=not significant. (E) Tumor growth of A2780 xenograft model treated with niraparib or olaparib at the maximum tolerated dose. Niraparib and olaparib were administered at 62.5 and 100 mg/kg daily, respectively. P value calculated by Student’s t test to compare niraparib and olaparib on day 9. (F) Table summarizing TGI and P value calculated by Student’s t test for niraparib or olaparib compared to vehicle on day 9, **P< 0.01, NS=not significant.