Table 2.
Ring opening of aziridiniums: Synthesis of optically active b-aminonitriles
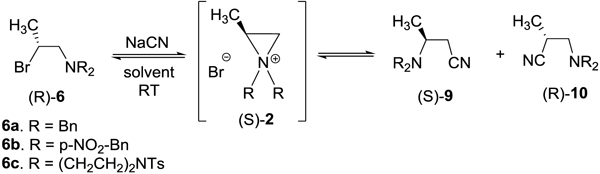 |
| entry | substrate | Nu | solvent | reaction time | product (yield%) | *ratio of (S)-9/(R)-10 | +ee (%) of (S)-9/(R)-10 |
|---|---|---|---|---|---|---|---|
| 1 | 6a | CN | CH3CN | 4 d | 9aa/10aa (82/6) | 14:1 | 97.4/ >99 |
| 2 | 6a | CN | DMSO | 30 min | 9aa/10aa (76/7) | 11:1 | 97.5/ >97.9 |
| 3 | 6a | CN | CH3CN/H2O | 10 min | 9aa/10aa (80/7) | 11:1 | 97.9/ 98.6 |
| 4 | 6b | CN | DMSO | 4 h | 9b (92.8) | >99 | |
| 5 | 6c | CN | CH3CN/H2O | 30 min | 9c (98.5) | >99 |
determined by isolated yield of the regioisomers by prep-TLC or flash LC.
determined by chiral HPLC.
