Table 6.
Optimized geometries and selected parameters of (S)-aziridinium ions 3a′, 5a′, 6a′, and 7a′ and transition states (TS) for formation of (R)-β-halo amines 3a, 5a, 6a, and 7a from ring opening reaction of aziridinium ion (Az+) with halide via a SN2 pathway (PBE0/cc-pVTZ).
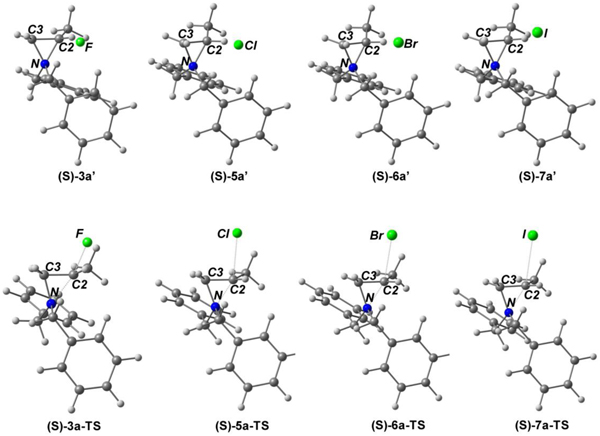 |
| parameter | (S)-3a′ (X =F) |
(S)-3a-TS (X = F) |
(S)-5a′ (X = Cl) |
(S)-5a-TS (X = Cl) |
(S)-6a′ (X = Br) |
(S)-6a-TS (X = Br) |
(S)-7a′ (X = I) |
(S)-7a-TS (X = I) |
|---|---|---|---|---|---|---|---|---|
| X-C2* | 2.610 | 2.184 | 3.184 | 2.705 | 3.351 | 2.846 | 3.591 | 3.068 |
| X-C3* | 3.228 | 2.931 | 3.434 | 3.086 | 3.564 | 3.217 | 3.779 | 3.445 |
| N-C2* | 1.527 | 1.813 | 1.517 | 1.693 | 1.516 | 1.715 | 1.515 | 1.749 |
| N-C3* | 1.487 | 1.474 | 1.494 | 1.485 | 1.494 | 1.482 | 1.494 | 1.477 |
| C2-C3* | 1.473 | 1.460 | 1.469 | 1.453 | 1.469 | 1.454 | 1.469 | 1.457 |
| X# | −0.75 | −0.68 | −0.82 | −0.82 | −0.82 | −0.80 | −0.84 | −0.79 |
| N# | −0.38 | −0.46 | −0.38 | −0.43 | −0.38 | −0.43 | −0.38 | −0.43 |
| C2# | −0.06 | −0.05 | −0.04 | 0.00 | −0.04 | 0.00 | −0.03 | 0.00 |
| C3# | −0.21 | −0.23 | −0.20 | −0.21 | −0.20 | −0.22 | −0.20 | −0.22 |
All bond lengths are in Angstrom.
Atomic charges are calculated within the NPA framework.
