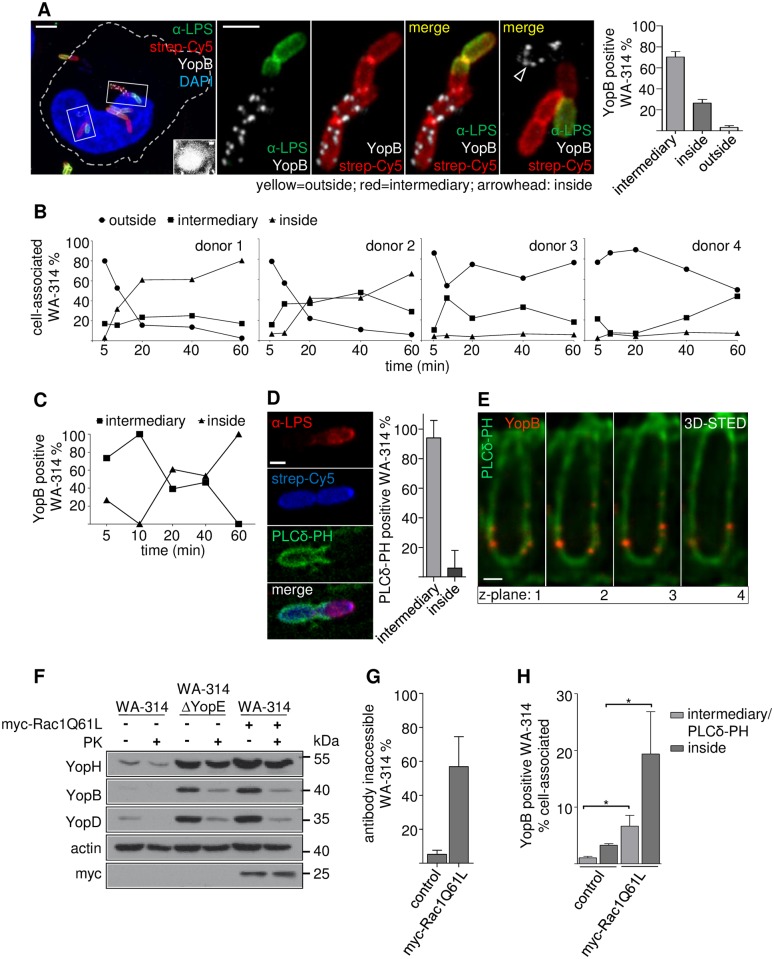
Fig 5. Formation of translocons is triggered in a prevacuole, a PI(4,5)P2 enriched intermediary host cell compartment.
(A) YopB positive bacteria reside in an intermediary compartment. HeLa cells were infected with surface-biotinylated WA-314 at a MOI of 20 for 60 min and were stained with anti-LPS antibody and streptavidin-Cy5 without cell permeabilization. Cells were permeabilized and immunostained with anti-YopB antibody and DAPI. From left to right: 1. Overview of infected HeLa cell with Yersinia in different stages of internalization. Inset shows strongly increased contrast settings to visualize the host cell body. 2.–4. Enlargements of a bacterial chain showing YopB staining only in the part of the chain located in the intermediary compartment (red in merge) but not in the outside location (yellow in merge). 5. Enlargement of a YopB positive bacterium (white) in the inside compartment (no red or green). Scale bars: 5 μm (overview) and 2 μm (enlargements). Bar diagram: Distribution of YopB positive bacteria between intermediary, inside and outside compartments. Bars represent mean ± S.D. of n = 95 YopB positive bacteria recorded in 2 independent experiments. (B) Bacterial passage from the outside into the intermediary and inside compartments of macrophages. Primary human macrophages of donors 1–4 were infected with biotinylated WA-314 at a MOI of 10 for the indicated time periods and stained with anti-LPS antibody, streptavidin-Cy5 (both without cell permeabilization) and DAPI to score their localization to the outside, inside or intermediary compartments as in (A). Data represent means of at least n = 1000 bacteria investigated per donor. (C) YopB positive bacteria passage from the intermediary to the inside compartment in macrophages. Experimental conditions as in (B) but with no LPS but YopB immunostaining. Data represent mean of n = 952 YopB positive bacteria investigated from 1 donor. (D) Intermediary compartment is marked by PLCδ-PH-GFP. HeLa cells expressing PLCδ-PH-GFP and myc-Rac1Q61L were infected with surface-biotinylated WA-314 at a MOI of 10 for 60 min, stained with anti-LPS antibody and streptavidin-Cy5. Bacteria with PLCδ-PH-GFP enrichment were judged for their localization as in (A). Scale bar: 1 μm. Bars represent mean ± S.D. of n = 398 PLCδ-PH-GFP positive bacteria investigated in 1 experiment. (E) YopB spots colocalize with the PLCδ-PH-GFP marker at STED resolution. HeLa cells expressing PLCδ-PH-GFP and myc-Rac1Q61L were infected with WA-314. Cells were co-immunostained for YopB (secondary antibody Abberior-StarRed) and GFP (AbberiorStar580). Z-stacks were recorded in 3D-STED mode and z-planes (distance: 90 nm) of YopB inserted in a PLCδ-PH-GFP enriched membrane are depicted. Scale bar: 0.5 μm (F) External addition of proteinase K to infected HeLa cells degrades YopB and YopD but not YopH. HeLa cells were infected with WA-314 or WA-314ΔYopE and myc-Rac1Q61L overexpressing HeLa cells were infected with WA-314 at a MOI of 100 for 60 min as indicated. Cells were incubated with PK for 20 min. PK was inactivated with PMSF, cells were extracted with digitonin and analyzed by Western blot for YopB and YopD, YopH (translocated effector protein), myc (myc-Rac1Q61L expression) and actin (loading control). Data are representative of 3 independent experiments. (G) Rac1Q61L overexpression increases uptake of WA-314 into HeLa cells. Control or myc-Rac1Q61L expressing HeLa cells were infected with WA-314 at a MOI of 20 for 60 min, fixed and stained with anti-LPS antibody (antibody accessible bacteria in outside location) and then permeabilized and stained with the same anti-LPS antibody in combination with a different secondary antibody (antibody inaccessible bacteria in intermediary and inside compartments). Bars represent mean ± S.D. of n = 1609 bacteria in control transfected cells and n = 3482 bacteria in myc-Rac1Q61L transfected cells investigated in 3 independent experiments. (H) Rac1L61 expression significantly increases the number of YopB positive bacteria in the intermediary and inside compartments. HeLa cells expressing PLCδ-PH-GFP and either empty vector (control) or myc-Rac1Q61L were infected with WA-314 at a MOI of 50 for 60 min and stained with anti-YopB antibody. The number of YopB positive bacteria as fraction of total cell associated bacteria and their distribution between the intermediary (PLCδ-PH-GFP positive) and inside (PLCδ-PH-GFP negative) compartments was determined. Bars represent mean ± S.D. of n = 1820 bacteria in control transfected cells and n = 6020 bacteria in myc-Rac1Q61L transfected cells investigated in 3 independent experiments.