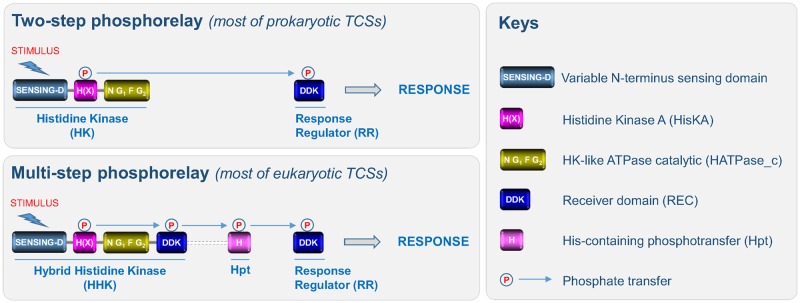
Fig. 1.
—Domain organization and TCSs signaling. The canonical structure of HKs is composed of: 1) a highly variable N-terminal sequence that determines which stimulus is perceived by the HK (sensing-D); 2) and both histidine kinase A (H(X)) (including a phosphorylatable histidine residue in the H-box and an X-box) and histidine kinase-like ATPase catalytic (including four distinct boxes: the N-, G1-, F-, and G2-boxes) subdomains. In prokaryotes, TCSs usually consist of a two-step phosphorelay between an HK and an RR. Perception of a stimulus induces autophosphorylation of the HK which thus acts as a primary sensor, and the phosphate is then transferred to an RR which acts as a transcription factor to directly regulate a series of genes required for an adapted response. Most eukaryotic HKs harbor an additional C-terminal receiver domain (REC) characterized by the presence of a three amino acid signature (DDK). Thus, eukaryotic HKs are generally called hybrid HKs (HHKs). In eukaryotes, TCSs are composed of additional modules, such as the Hpt domain and the signaling routes that mediate the perception of stimuli correspond to a multistep phosphorelay between three families of proteins (HHK, Hpt, and RR). In most eukaryotic TCSs, the RR either acts directly as a transcription factor or governs downstream elements (MAPK cascades, the cAMP pathway, or Raf-like kinases). Note that in a few HKs, the Hpt domain is fused to the REC domain.