Abstract
Screening for fragile X syndrome (FXS) is essential in children with developmental delay or intellectual disability (ID). In addition, using clinical screening checklists remains of high interest in resource-limited settings. We aimed to gain insight into the prevalence of FXS and the distribution of CGG alleles and to evaluate the usefulness of three checklists in specialized institutions in Kinshasa, DR Congo. We recruited 80 males and 25 females from six specialized institutions in Kinshasa and administered a questionnaire comprising items from the following FXS checklists: Hagerman, Maes, and Guruju. FMR1 CGG repeats were assessed for every patient. About 37% of patients were referable for FX testing based on Hagerman’s checklist, 35% for Maes’, and 43.80% for Guruju’s, but none of them was molecularly confirmed to have FXS. Thus, specificities were 62.86, 64.76, and 56.5%, respectively, for Hagerman, Maes, and Guruju, respectively. The mean CGG allele size was 28.55 ± 2.83 (ranges, 17–48). The 29 CGG was the most frequent allele (24.61%). Thus, existing checklists should not be automatically applied to Congolese patients without adjustments. The distribution of CGG repeats and the number of CGG alleles are similar to other African studies.
Electronic supplementary material
The online version of this article (10.1007/s12687-018-0374-4) contains supplementary material, which is available to authorized users.
Keywords: Fragile X syndrome, Checklist, Behavioral manifestation, Dysmorphism, Democratic Republic of Congo
Introduction
The fragile X syndrome (FXS) (OMIM 300624) is one of the most common monogenic causes of intellectual disability (ID). Its prevalence varies between 1/6000 and 1/4000 males in the general population (de Vries et al. 1999; Turner et al. 1996). Because of this high incidence, screening for fragile X syndrome is considered essential in any child with developmental delay or intellectual disability (ID) (Moeschler and Shevell 2014). A variable prevalence is observed in different geographical parts of the world, but prevalence also depends on the clinical characteristics of the tested group, gender, and sampling strategy (Crawford et al. 2001). Only few studies have widely investigated FXS in Africa. In children attending schools for special education, Goldman reported a prevalence of 6.1% of FXS among black Africans in South Africa (Goldman et al. 1998). Later, Crawford et al. reported a prevalence of 1/2545 among African-Americans in special education in the public school system of metropolitan Atlanta, USA (Crawford et al. 2002). To date, the prevalence of FXS was not investigated in specialized schools in DR Congo even if few cases have recently been reported (Lubala et al. 2018).
In at least 99% of FXS cases, the mutation is an expansion of the CGG repeats in the 5′-UTR in the promoter region of the FMR1 (fragile X mental retardation 1). Normal alleles range from ~ 5 to ~ 44 repeats, intermediate alleles from ~ 45 to ~ 54 repeats, premutation alleles from ~ 55 to ~ 200 repeats, and full mutations over 200–230 repeats (Maddalena et al. 2001; Monaghan et al. 2013). Comparatives studies between African-Americans and Caucasian Americans reported a significant difference in the distribution of common alleles with shorter alleles (20–23 repeats) as well as larger alleles (41–60 repeats) being less frequent in African-Americans (Crawford et al. 2000; Eichler et al. 1995). Although the 29 and 30 CGG repeats are the most prevalent alleles in Africans, African-Americans, and Caucasians, the 29 repeats allele has a significantly higher frequency in black Africans whereas the 30 repeat allele is significantly more frequent in white Africans (Chiurazzi et al. 1996; Crawford et al. 2002; Essop and Krause 2013).
The FXS is associated with various clinical presentations depending on the number of repeats, sex, and age (Oostra and Willemsen 2009). Hence, concepts such as “Fragile X Spectrum Disorder” (FXSD) or FMR1-related disorders have been proposed in order to include all FMR1-related phenotypes (Lozano et al. 2014). The full mutation in young children manifests often with attention deficit hyperactivity disorder (ADHD) and/or autism spectrum disorders (ASD) (Gabis and Kesner 2007; Hagerman 2006; Sullivan et al. 2006). In older children, adolescents, and adults, the classic presentation consists of intellectual disability with an IQ < 50, associated to more characteristic behavioral and dysmorphic features (D’Hulst and Kooy 2009; Hagerman et al. 1991; Lachiewicz et al. 2000; Lozano et al. 2014; Mirkin 2007; Tuncbilek et al. 1999). Although none of the listed features is pathognomonic for FXS, their combination has been used as checklists to clinically screen for FXS (Butler et al. 1991; Lachiewicz et al. 2000; Laing et al. 1991). These checklists are not intended to substitute molecular testing. Instead, they allow a fast and efficient selection of patients with a higher probability for having FXS (Behery 2008; de Vries et al. 1999; Hagerman et al. 1991).
So far, no checklist has specifically been designed or adapted for the black African population. Indeed, craniofacial features in a population of African origin may show critical differences compared to those in Caucasian, as it was reported for the 22q11.2 deletion syndrome (McDonald-McGinn et al. 2005) and for FXS (Lubala et al. 2018; Schwartz et al. 1988). Such clinical variability points to the necessity to design population-specific checklists or adapt existing tools to the population of interest. Examples of population-specific checklists for FXS have been proposed for the Indian and Turkish population (Guruju et al. 2009; Tuncbilek et al. 1999).
The present study aimed to gain insight into the prevalence of FXS and the distribution of CGG alleles and to evaluate the usefulness of three selected checklists as a screening tool for FXS in patients with ID followed in specialized clinics and schools in Kinshasa, DR Congo.
Material and methods
Patients
We recruited 127 index patients from two hospitals and four schools specialized for ID across Kinshasa in the Democratic Republic of Congo (DRC). Informed consent was obtained from parents or legal representatives. We identified clinically recognizable syndrome in 22 patients. This study concerns the 105 patients (80 males and 25 females) remained without clinical diagnostic. Ages ranged from 1.24 to 24.65 years (average 10 ± 4.7 years). More than 73% of patients were aged between 5 and 10 years. We performed a standardized clinical examination including measurements and evaluation of dysmorphism. Personal and family histories were obtained from the parents or legal representatives. A positive familial history of ID was defined as the presence of relative with ID up to the 3rd degree.
We designed a research questionnaire (Addendum 1) containing items from three existing FXS checklists. A short explanation was provided for each item. We used the checklist from Hagerman (Hagerman et al. 1991), from Maes (Maes et al. 2000), and from Guruju (Guruju et al. 2009). The checklist from Hagerman was selected because it is the most widely used, and also, because it was the basis for most others (Guruju et al. 2009; Tuncbilek et al. 1999). The Maes checklist presents the advantage of taking into account prediction power of each item. They attributed coefficients to the most predictive items, thus increasing the chance for a fragile X patient to rank at the top. We also included the checklist from Guruju because it added two additional morphological features.
One week before the interview, the research questionnaire was sent to the parents to make them familiar with the questions and allow them to prepare their answers. On the clinical examination day, A.L. personally scored the questionnaires. Parents or legal representatives were asked to respond with YES when they were certain that the item described in the questionnaire was currently present or had been present in the patient, NO when they were convinced that the item had never been observed or NOT SURE when they were uncertain. These responses were then translated into scores according to the scoring rules specific for each of the three checklists. Hagerman’s checklist has three scales, and thus, YES was scored as 2, NOT SURE as 1, and NO as 0. Guruju and Maes were 2-scale tools where YES received the score 1 whereas NOT SURE and NO received the score 0. The threshold score is the minimal cumulative that was showed to be predictive for FXS in the original study. In this study, when a patient obtained, for a particular checklist, a cumulative score equal or above the threshold score for that checklist, he was counted as fragile X positive for that checklist. Threshold scores are ≥ 10 for Hagerman, ≥ 17 for Maes, and ≥ 5 Guruju. We wanted to use item frequencies to determine the most prevalent features in our patient cohort and the cumulative scores to assess the sensitivity and specificity of each checklist, depending on the results from molecular testing.
DNA extraction
Venous blood was sampled from peripheral vein, and genomic DNA was extracted by the salt saturation method as previously described (Miller et al. 1988). Between 2010 and 2012, DNA extraction was performed in the Centre for Human Genetic at Leuven, Belgium. Then, from 2013 onwards, a DNA extraction facility was implemented at the Institut National de Recherche Biomédicale (INRB) in Kinshasa.
FMR1 CGG repeats testing
To assess the fragment length of the CGG repeats in the 5′UTR region of the FMR1 gene, we amplified the target region during a PCR reaction using the PRC-enhancer kit (Invitrogen) and the FRAXA-A and FRAXA-B primers.
Primer sequences:
FRAXA-A: GAC GGA GGC GCC GCT GCC AGG6FAM
FRAXA-B: GTG GGC TGC GGG CGC TCG AGG
The reaction mix contained 5.6 μl of water, 2 μl of the 10× PCRx Amplification buffer, 0.6 μl of 50 mM MgSO4, 1 μl of dNTPs (4 mM), 8 μl of PCRx Enhancer Solution, 1.5 μl of Primer mix (10 pm/μl), and 0.25 μl of Taq DNA Polymerase from Roche. The cycling comprised an initial denaturation at 95 °C for 3 min followed by 27 amplification cycles including short denaturation at 95 °C for 15 s, annealing at 64 °C for 1 min and elongation at 75 °C for 1 min. The reaction was terminated with a final elongation at 75 °C for 7 min, and the cooling to 15 °C/∞. PCR product was controlled on 2% agarose gel with 1-kb size marker. Then, 2 μl of the remaining PCR product was resuspended with an admixture of 20 μl HiDi Formamide (Applied Biosystems) and Rox 500 (Applied Biosystems). Fragments were separated on the on ABI 3730xl DNA Analyzer (Applied Biosystems, Foster City, CA, 94404, USA) then analyzed with GeneMapper® Software 5 (Life Technologies Ltd., Paisley, UK).
Results
FXS clinical checklists
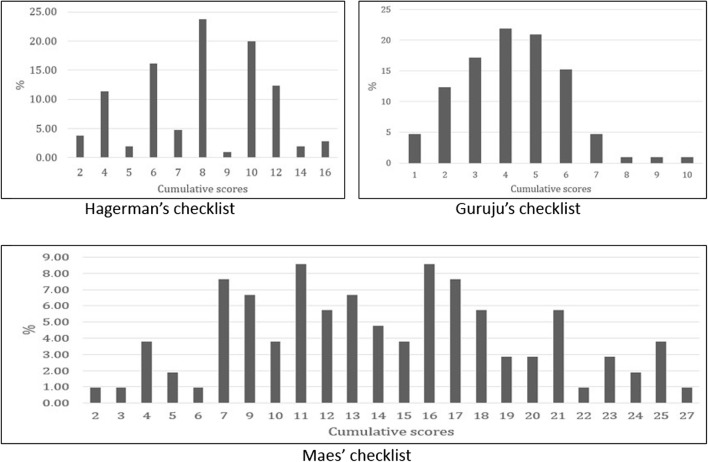
Mean cumulative scores were 8.13 ± 3.07 (ranges 2–16), 14.30 ± 5.92 (ranges 2–27), and 4.24 ± 1.75 (ranges 1–10) for the Hagerman, Maes, and Guruju screening tools, respectively (Fig. 1). Thirty-nine patients (37.14%) showed a total equal to or higher than the threshold score for Hagerman’s tool versus 37 patients (35.24%) for Maes’ and 46 patients (43.80%) for Guruju’s.
Fig. 1.
Distribution of cumulative scores for the three checklists. More than one third of patients had cumulative scores above the threshold score of 10 for Hagerman, 17 for Maes, and 5 for Guruju
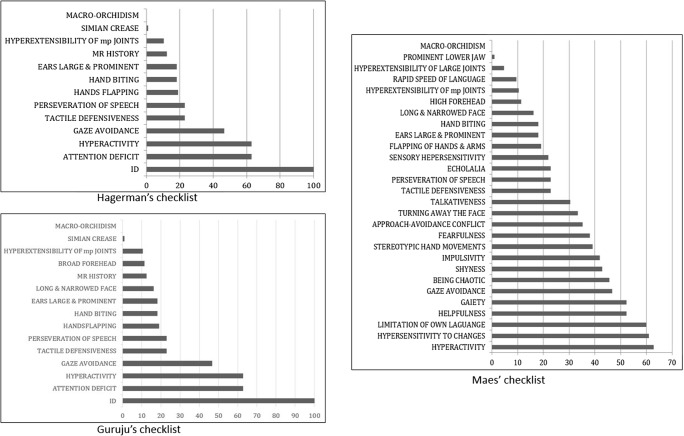
In addition to the ID, behavioral features were more prevalent in all three tools compared to morphological signs. In particular, for the checklist from Hagerman and the one from Guruju, attention deficit and hyperactivity were the most prevalent items (62.86%) followed by gaze avoidance (46.67) (Fig. 2). The most prevalent morphological trait, the large and prominent ears, was observed in 18.09% followed by simian crease (0.95%). Likewise, hyperactivity prevailed in Maes’s checklist with hypersensitivity to change as the second common item (60.95%), contrasting to large and prominent ears observed in only 18.09%.
Fig. 2.
Distribution of items in the three checklists. Hyperactivity was invariably far more prevalent than morphological signs in the three checklists
The average number of behavioral items per patient was 2.55 with Hagerman’s and Guruju’s tools and 7.79 items with Maes’ tool. Conversely, the average number of physical features per patient was 00.29 for Hagerman, 0.57 for Guruju, and 0.62 for Maes.
CGG allele sizes
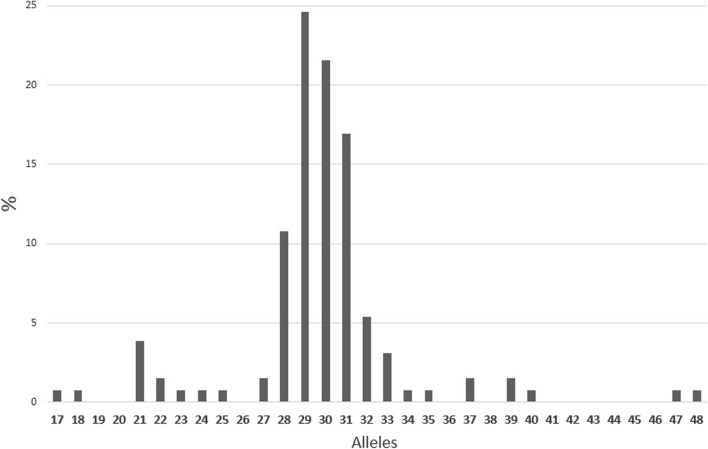
We analyzed a total of 130 chromosomes including 80 from the males and 50 from the 25 females. Twenty-one different alleles were observed. The mean allele size was 28.55 ± 2.83 (ranges, 17–48) (Fig. 3). The most frequent allele size was 29 (24.61%), and 73.84% of alleles spanned between 28 and 31 repeats. There was a second and small peak at 21 repeats. No full mutation or premutation were detected. Two males carried an intermediate allele of 47 and 48 repeats. The 47 repeat allele was inherited from the normal mother who had highly skewed X-chromosome inactivation (92.75/7.25%). DNA from parents of the patient with 48 repeats was not available.
Fig. 3.
Distribution different CGG alleles. Only 21 alleles were observed including two intermediate alleles, and there was a clustering around the mean
Sensitivity and specificity of the checklists
Given the absence of molecularly confirmed fragile X patients in our cohort, sensitivity could not be calculated for the three checklists. However, we calculated the specificity, using the referral thresholds and found 62.86, 64.76, and 56.5%, respectively, for Hagerman, Maes, and Guruju (Table 1). The checklist from Maes exhibited the highest specificity of the three.
Table 1.
Specificities in this study versus the original studies
| Checklists | Referral threshold | This study (%) | Original studies (%) |
|---|---|---|---|
| Hagerman | ≥ 10 | 62.86 | 87 |
| Maes | ≥ 17 | 64.76 | 92.3 |
| Guruju | > 5 | 56.5 | 73.18 |
Discussion
The fragile X syndrome is considered to be one of the most common causes of ID worldwide. However, no data exist on the incidence of FXS in Central Africa. This is the first study to evaluate this question in a systematic way in a high-risk population of individuals attending schools for special education or specialized clinics for persons with ID in DR Congo.
Among 105 individuals, 80 males and 25 females, not a single case of FXS was diagnosed by molecular testing. This may seem in contradiction with the observations made in South Africa where Goldman et al. reported a prevalence of 6.1% among black patients in specialized schools, and the results of Essop and Krause who identified FXS in 5.2% in black patients with ID tested in a period of 25 years in Johannesburg (Essop and Krause 2013; Goldman et al. 1998). The absence of positive cases in our study in specialized institutions may be explained by certain limitations of this study. One of the limitations is the relatively small size of studied sample. The second important limitation would be the limited resources in this country. It should be remembered that DR Congo is a low-income country as classified by the World Bank, and the average size of a family is 5.3 according to the nationwide health inquiry conducted between 2003 and 2004 (https://www.dhsprogram.com/pubs/pdf/FR300/FR300.pdf, last accession was on 6 May 2018). Although reliable or consistent data on wages in DR Congo are not available, as in many other developing countries, it is commonly considered that people live with less than 1 US$ a day. In this context, the average 50 US$ a month per child that is charged by the specialized schools in Kinshasa may seem prohibitive for most of the families. Thus, not all parents can afford paying the specialized institutions, and the majority of families are forced to keep their children at home, especially when they have severe behavioral manifestations or disabilities. Consequently, only patients from families with high revenues as well as those with less severe impairments are encountered in these institutions. Finally, the education level of parents could also be a limitation since only educated people can understand the usefulness of specialized and expensive schools. The nationwide health inquiry of 2003–2004 also informed that only 5 and 10% of Congolese women and men, respectively, have completed at least the secondary school, suggesting a very low literacy level in the country. Although we did not specifically question parents on their education level, we can assume that majority of parents of patients recruited in this study was among the elite since they were all able to fluently communicate in French, the official language in the education and administration system in DR Congo. This may have resulted in an underrepresentation of FXS cases in this study. Although it may seem less likely, FXS patients in DR Congo may present differently and thus be mainly referred to different specialties such as psychiatry or speech therapy.
However, given the presence of intermediate alleles in our population (2/130 chromosomes), and the recent report of FXS patients from Lubumbashi, the second biggest city in DR Congo, we can anticipate that FXS is very likely to be present in Kinshasa. In order to increase the chance to identify FXS patients, future studies should include a larger cohort of individuals, consider different environments (schools and neighborhood survey), and work in collaboration with various medical specialties (psychiatry, neurology, pediatrics etc.). In addition, the recruitment of familial cases, with a pedigree compatible with X-linked inheritance, may increase the likelihood of identifying FXS cases.
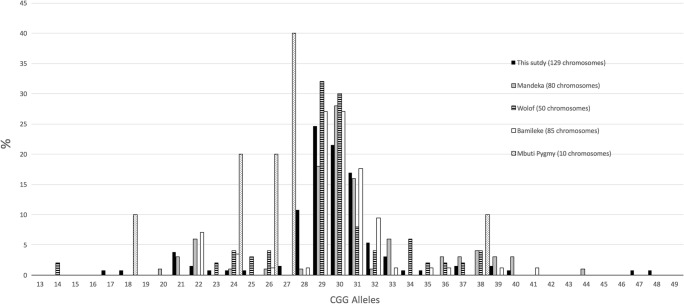
We have observed only 21 different alleles among the 130 studied chromosomes (Fig. 3). Crawford et al. reported a larger number of alleles in Caucasians (42 alleles) compared to African-Americans (37 alleles) (Crawford et al. 2000). The number of distinct alleles in the Congolese is similar to those reported in other African groups (Fig. 4): 17 for Senegalese Mandeka (Kunst et al. 1996), 14 in Senegalese Wolof, 13 in Cameroonian Bamileke (Chiurazzi et al. 1996), and 10 for Mbuti Pygmies from DR Congo (Eichler and Nelson 1996).
Fig. 4.
Comparison of CGG distribution to four other African groups. Limitation in allele numbers in the four groups: the similar distribution for Mandeka, Wolof, Bamileke, and this study but the absence of many more alleles in Mbuti pygmies
Alleles in our cohort ranged from 17 to 48 repeats (mean, 28.55 ± 2.83). Interestingly, about 74% were between 28 and 31 repeats and we detected only two alleles shorter than 20 repeats and two larger than 45 (Fig. 3). This observation is consistent with the prior observation that shorter and larger alleles are rare in African-Americans and Africans (Crawford et al. 2000; Peprah et al. 2010). The mean repeat size in this study (28.55) is similar to the literature reports (Maddalena et al. 2001; Peprah et al. 2010; Tuncbilek et al. 1999; Tzeng et al. 1999; Van Esch 2006; Yim et al. 2008). Alleles in our cohort delineate two clusters. A major cluster around the mean, and a minor cluster at 21 repeats. A similar two-peak pattern has been previously reported in African as well as non-African populations (Chiurazzi et al. 1996; Crawford et al. 2000; Kunst et al. 1996; Peprah et al. 2010).
After the results from molecular testing of FMR1 were available, we evaluated the assessment of the three selected checklists knowing that there was no true positive patient in our cohort. The clinical scoring tools indicate a higher frequency for behavioral characteristics compared to physical features. Attention deficit and hyperactivity were the most common items. This may be ascribed to the younger age of patients. It may also be that parents or tutors tend to overrate these features. Importantly, our study suggests that these behavioral characteristics are so common in non-FXS children with ID, and therefore, incorporating these behavioral features in FXS screening lists will result in low specificities, at least at the thresholds used in the original screening tools. However, the predictive power of each feature can only be determined in a population-specific case control study. Overall, we can anticipate that the three checklists have poor performances on Congolese population. An adaptation might be necessary to improve the performance in Congo.
Raw data and DNA are available on demand.
Electronic supplementary material
(PDF 172 kb)
Acknowledgements
The authors are grateful to the laboratory of Prof Gert Matthijs; to Bijou Myndyo, Ervie-Winner, Eben and Etsa-Edi Lumaka, Cathy Nkunku, and Dr. Gerrye Mubungu for their strong contribution and support to this work.
Funding information
A.L. acknowledges the contribution of the Research Foundation - Flanders (FWO) travel grants for research abroad (ref: V405213N and K210115) to this research.
Compliance with ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000(5). Informed consent was obtained from all patients for being included in the study.
Our research protocol was approved under the number ESP/CE/008/2015 by the National Ethical Committee of the Public Health School of the University of Kinshasa, Kinshasa, DR Congo.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Behery AK. Fragile x-syndrome: clinical and molecular studies. J Egypt Public Health Assoc. 2008;83:273–283. [PubMed] [Google Scholar]
- Butler MG, Mangrum T, Gupta R, Singh DN. A 15-item checklist for screening mentally retarded males for the fragile X syndrome. Clin Genet. 1991;39:347–354. doi: 10.1111/j.1399-0004.1991.tb03041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiurazzi P, Destro-Bisol G, Genuardi M, Oostra BA, Spedini G, Neri G. Extended gene diversity at the FMR1 locus and neighbouring CA repeats in a sub-Saharan population. Am J Med Genet. 1996;64:216–219. doi: 10.1002/(SICI)1096-8628(19960712)64:1<216::AID-AJMG39>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Crawford DC, Zhang F, Wilson B, Warren ST, Sherman SL. Fragile X CGG repeat structures among African-Americans: identification of a novel factor responsible for repeat instability. Hum Mol Genet. 2000;9:1759–1769. doi: 10.1093/hmg/9.12.1759. [DOI] [PubMed] [Google Scholar]
- Crawford DC, Acuna JM, Sherman SL (2001) FMR1 and the fragile X syndrome: human genome epidemiology review. Genet Med 3:359–371. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4493892/ [DOI] [PMC free article] [PubMed]
- Crawford DC, et al. Prevalence of the fragile X syndrome in African-Americans. Am J Med Genet. 2002;110:226–233. doi: 10.1002/ajmg.10427. [DOI] [PubMed] [Google Scholar]
- D’Hulst C, Kooy RF. Fragile X syndrome: from molecular genetics to therapy. J Med Genet. 2009;46:577–584. doi: 10.1136/jmg.2008.064667. [DOI] [PubMed] [Google Scholar]
- de Vries BB, et al. Screening for the fragile X syndrome among the mentally retarded: a clinical study. The Collaborative Fragile X Study Group. J Med Genet. 1999;36:467–470. [PMC free article] [PubMed] [Google Scholar]
- Eichler EE, Nelson DL. Genetic variation and evolutionary stability of the FMR1 CGG repeat in six closed human populations. Am J Med Genet. 1996;64:220–225. doi: 10.1002/(SICI)1096-8628(19960712)64:1<220::AID-AJMG40>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Eichler EE, Hammond HA, Macpherson JN, Ward PA, Nelson DL. Population survey of the human FMR1 CGG repeat substructure suggests biased polarity for the loss of AGG interruptions. Hum Mol Genet. 1995;4:2199–2208. doi: 10.1093/hmg/4.12.2199. [DOI] [PubMed] [Google Scholar]
- Essop FB, Krause A. Diagnostic, carrier and prenatal genetic testing for fragile X syndrome and other FMR-1-related disorders in Johannesburg, South Africa: a 20-year review. S Afr Med J. 2013;103:994–998. doi: 10.7196/SAMJ.7144. [DOI] [PubMed] [Google Scholar]
- Gabis L, Kesner Y. Behavioral characteristics of children with fragile X syndrome. Harefuah. 2007;146:469–474. [PubMed] [Google Scholar]
- Goldman A, Jenkins T, Krause A. Molecular evidence that fragile X syndrome occurs in the South African black population. J Med Genet. 1998;35:878. doi: 10.1136/jmg.35.10.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guruju MR, et al. Assessment of a clinical checklist in the diagnosis of fragile X syndrome in India. J Clin Neurosci. 2009;16:1305–1310. doi: 10.1016/j.jocn.2008.12.018. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ. Lessons from fragile X regarding neurobiology, autism, and neurodegeneration. J Dev Behav Pediatr. 2006;27:63–74. doi: 10.1097/00004703-200602000-00012. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, Amiri K, Cronister A. Fragile X checklist. Am J Med Genet. 1991;38:283–287. doi: 10.1002/ajmg.1320380223. [DOI] [PubMed] [Google Scholar]
- Kunst CB, Zerylnick C, Karickhoff L, Eichler E, Bullard J, Chalifoux M, Holden JJ, Torroni A, Nelson DL, Warren ST. FMR1 in global populations. Am J Hum Genet. 1996;58:513–522. [PMC free article] [PubMed] [Google Scholar]
- Lachiewicz AM, Dawson DV, Spiridigliozzi GA. Physical characteristics of young boys with fragile X syndrome: reasons for difficulties in making a diagnosis in young males. Am J Med Genet. 2000;92:229–236. doi: 10.1002/(SICI)1096-8628(20000605)92:4<229::AID-AJMG1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Laing S, Partington M, Robinson H, Turner G. Clinical screening score for the fragile X (Martin-Bell) syndrome. Am J Med Genet. 1991;38:256–259. doi: 10.1002/ajmg.1320380219. [DOI] [PubMed] [Google Scholar]
- Lozano R, Rosero CA, Hagerman RJ. Fragile X spectrum disorders. Intractable Rare Dis Res. 2014;3:134–146. doi: 10.5582/irdr.2014.01022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubala TK et al (2018) Fragile X syndrome with mosaic size mutation in a Bantu patient from Central Africa. Clin Dysmorphol. 10.1097/MCD.0000000000000208 [DOI] [PubMed]
- Maddalena A, et al. Technical standards and guidelines for fragile X: the first of a series of disease-specific supplements to the Standards and Guidelines for Clinical Genetics Laboratories of the American College of Medical Genetics. Quality Assurance Subcommittee of the Laboratory Practice Committee. Genet Med. 2001;3:200–205. doi: 10.1097/00125817-200105000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes B, Fryns JP, Ghesquiere P, Borghgraef M. Phenotypic checklist to screen for fragile X syndrome in people with mental retardation. Ment Retard. 2000;38:207–215. doi: 10.1352/0047-6765(2000)038<0207:PCTSFF>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- McDonald-McGinn DM, et al. The 22q11.2 deletion in African-American patients: an underdiagnosed population? Am J Med Genet A. 2005;134:242–246. doi: 10.1002/ajmg.a.30069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirkin SM. Expandable DNA repeats and human disease. Nature. 2007;447:932–940. doi: 10.1038/nature05977. [DOI] [PubMed] [Google Scholar]
- Moeschler JB, Shevell M. Comprehensive evaluation of the child with intellectual disability or global developmental delays. Pediatrics. 2014;134:e903–e918. doi: 10.1542/peds.2014-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan KG, Lyon E, Spector EB. ACMG Standards and Guidelines for fragile X testing: a revision to the disease-specific supplements to the Standards and Guidelines for Clinical Genetics Laboratories of the American College of Medical Genetics and Genomics. Genet Med. 2013;15:575–586. doi: 10.1038/gim.2013.61. [DOI] [PubMed] [Google Scholar]
- Oostra BA, Willemsen R. FMR1: a gene with three faces. Biochim Biophys Acta. 2009;1790:467–477. doi: 10.1016/j.bbagen.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peprah EK, Allen EG, Williams SM, Woodard LM, Sherman SL. Genetic diversity of the fragile X syndrome gene (FMR1) in a large sub-Saharan West African population. Ann Hum Genet. 2010;74:316–325. doi: 10.1111/j.1469-1809.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, et al. Fragile X syndrome: incidence, clinical and cytogenetic findings in the black and white populations of South Carolina. Am J Med Genet. 1988;30:641–654. doi: 10.1002/ajmg.1320300165. [DOI] [PubMed] [Google Scholar]
- Sullivan K, Hatton D, Hammer J, Sideris J, Hooper S, Ornstein P, Bailey D., Jr ADHD symptoms in children with FXS. Am J Med Genet A. 2006;140:2275–2288. doi: 10.1002/ajmg.a.31388. [DOI] [PubMed] [Google Scholar]
- Tuncbilek E, Alikasifoglu M, Boduroglu K, Aktas D, Anar B. Frequency of fragile X syndrome among Turkish patients with mental retardation of unknown etiology. Am J Med Genet. 1999;84:202–203. doi: 10.1002/(SICI)1096-8628(19990528)84:3<202::AID-AJMG6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Turner G, Webb T, Wake S, Robinson H. Prevalence of fragile X syndrome. Am J Med Genet. 1996;64:196–197. doi: 10.1002/(SICI)1096-8628(19960712)64:1<196::AID-AJMG35>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Tzeng CC, Cho WC, Kuo PL, Chen RM. Pilot fragile X screening in normal population of Taiwan. Diagn Mol Pathol. 1999;8:152–156. doi: 10.1097/00019606-199909000-00008. [DOI] [PubMed] [Google Scholar]
- Van Esch H. The fragile X premutation: new insights and clinical consequences. Eur J Med Genet. 2006;49:1–8. doi: 10.1016/j.ejmg.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Yim SY, Jeon BH, Yang JA, Kim HJ. Fragile X syndrome in Korea: a case series and a review of the literature. J Korean Med Sci. 2008;23:470–476. doi: 10.3346/jkms.2008.23.3.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 172 kb)