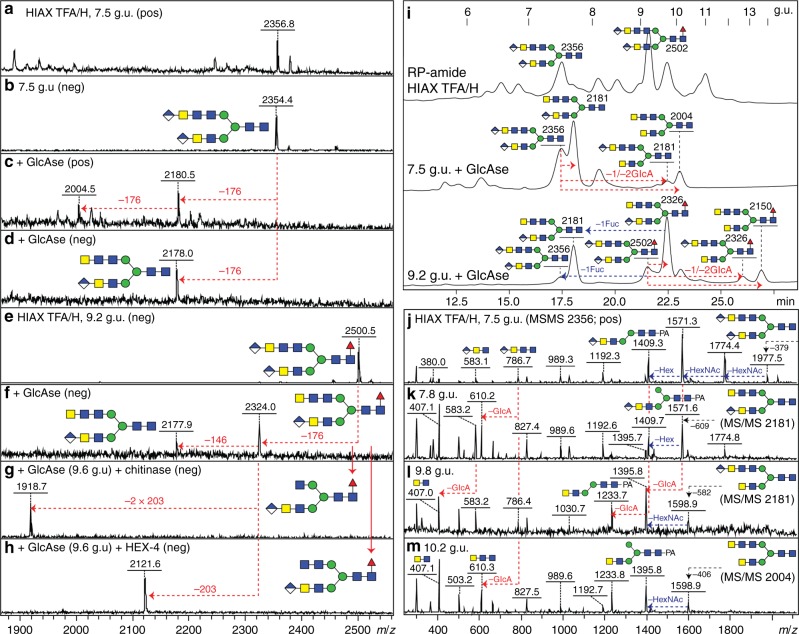
Fig. 8.
Effect of glucuronidase treatment on diglucuronylated N-glycans. a–f Two RP-amide HPLC example fractions from anionic HIAX pool H (see Fig. 5) were treated for 75 min with a commercial H. pomatia β-glucuronidase (GlcAse) prior to re-analysis by MALDI-TOF–MS (see also chromatograms in i) showing removal or one or two glucuronic acid residues (losses of 176 Da) as well as, due to an impurity in the enzyme preparation, partial defucosylation (loss of 146 Da). g, h Subsequent chitinase and HEX-4 treatment of the underlying exposed HexNAc3 motif followed by negative mode MALDI-TOF–MS reveals that the glucuronic acid on the upper antenna substitutes a GalNAcβ1,4GlcNAcβ1,4GlcNAc motif, which correlates also with LC–MSn data (see Fig. 7). i Removal of glucuronic acid and fucose (as defined by changes in m/z as shown in c, d and f) correlates with shifts in RP-amide HPLC elution time as compared to the original chromatogram for anionic HIAX pool H (see arrows showing the forward shift caused by defucosylation (blue) and the backward shift after deglucuronylation (red), which is larger when the GlcA residue is lost from the lower arm); structures are annotated with m/z values for the [M+H]+ ions. j–m Positive mode MALDI-TOF–MS/MS of the 7.5 g.u. glycan before and after glucuronidase treatment revealing differences in fragmentation between the original glycan (j) and the partially (k and l) and fully deglucuronylated (m) products of different elution times; key changes in the B- and Y-fragments are indicated (e.g. loss of B-ions at m/z 583 and 786, appearance of ones lacking glucuronic acid at m/z 407 and 610 and shifts in the pyridylamino-containing Y-ions). For the MS/MS of the untreated and treated forms of the m/z 2502 glycan (refer to Fig. 6k and Supplementary Figure 15)