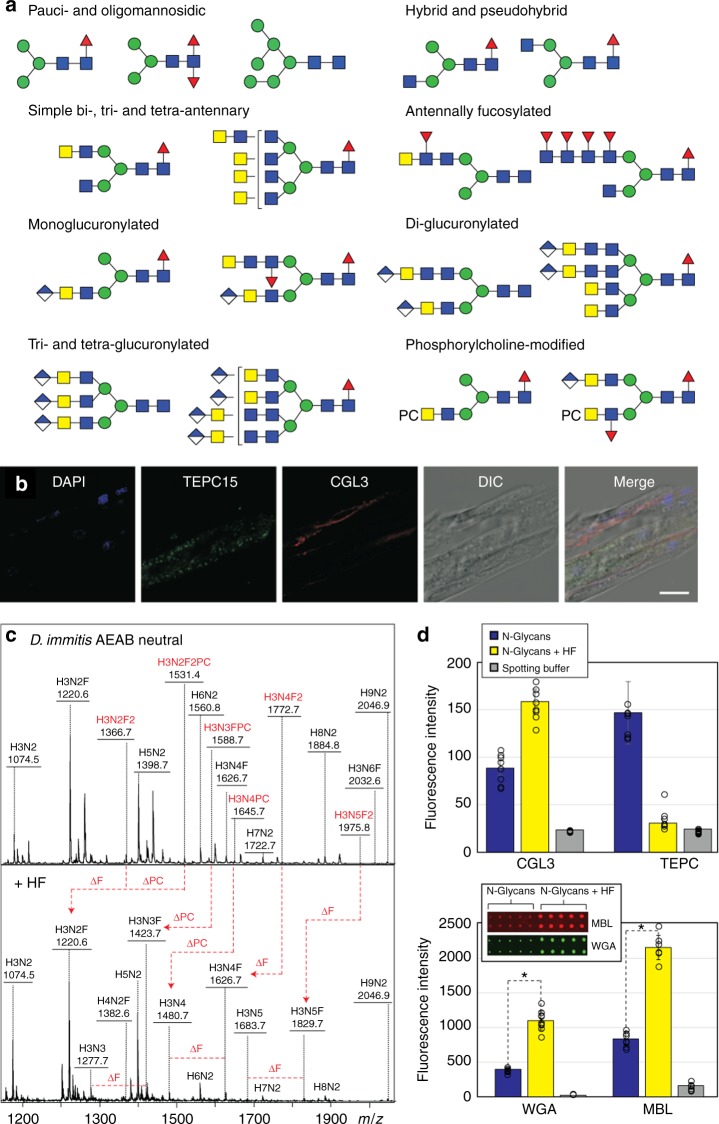
Fig. 9.
Glycan epitopes in D. immitis. a A summary of neutral and anionic N-glycan structures highlighting major antennal modifications; for a fuller set of defined structures refer to Supplementary Table 3. b The presence of HexNAc and phosphorylcholine residues in L3 larvae was probed using CGL3 (red) and TEPC 15 (green) by indirect fluorescence microscopy; also shown are the DAPI staining (for DNA) and DIC (differential interference contrast) images and the scale bar corresponds to 10 µm. c MALDI-TOF–MS of the AEAB-labelled neutral N-glycome before and after hydrofluoric acid treatment indicating loss of antennal fucose and phosphorylcholine residues. d The binding of Coprinopsis galectin (CGL3; 10 µg/ml), wheat germ agglutinin (WGA; 10 µg/ml) and human mannose binding lectin (MBL) to the Dirofilaria glycans increases after hydrofluoric acid treatment, while that to anti-phosphorylcholine (TEPC 15 IgA monoclonal) decreases. The charts indicate the uncorrected fluorescence values with the standard deviations (mean of 10 spots; analysed with an unpaired two-tailed parametric t-test with a 95% confidence level) as well as a negative control (spotting buffer only; example array scans are shown for the WGA and MBL data). Note that previous data indicate that CGL3 can recognise LacdiNAc, whereas WGA is known to bind HexNAcn motifs and MBL to a wide range of glycans including those with terminal mannose or N-acetylglucosamine residues. Refer to Supplementary Figures 16–19 and 21 for (i) western blotting and further micrograph data (including controls) on epitopes recognised by CGL3 and TEPC 15, (ii) a summary of MS data on proteins affinity purified on CGL3- and TEPC 15-Sepharose, (iii) further MBL binding experiments (before and after endoglycosidase H treatment) to the immobilised glycome pools and fractionated immobilised glycans as well as data on other lectin interactions to the natural glycans and to defined di- and tri-saccharides