Abstract
Despite the arrival of novel therapies, multiple myeloma (MM) remains incurable and new treatment options are needed. Chimeric antigen receptor (CAR) T cells are genetically modified T cells that express a CAR directed against specific tumour antigens. CAR T cells are able to kill target tumour cells and may result in long-lasting immune responses in vivo. The rapid development of CAR technologies has led to clinical trials in haematological cancers including MM, and CAR T cells might evolve into a standard treatment in the next few years. Only small patient cohorts with relapsed or refractory disease have so far been investigated, but promising preliminary results with high response rates have been obtained in phase I clinical trials with B cell maturation antigen (BCMA), CD19, CD38 and κ-light-chain CAR T cells. Additional preclinical studies on CD38 and SLAMF7-CAR T cells in MM treatment yielded preclinical results that merit further investigation. Beyond the T cell approach, recent studies have focussed on CAR natural killer (NK) cells in order to increase the reactivity of these effector cells. Finally, to investigate the targeting of intracellular antigens, cellular therapies based on engineered T cell receptors (TCRs) are in development. In this review, we discuss results from preclinical and early-phase clinical trials testing the feasibility and safety of CAR T cell administration in MM, as well as early studies into approaches that utilise CAR NK cell and genetically modified TCRs.
Subject terms: Myeloma, Molecular medicine, Myeloma
Introduction
With an incidence of approximately 5 cases per 100,000 persons per year, multiple myeloma (MM) accounts for around 10% of all haematological malignancies.1 Introduction of novel agents, such as thalidomide, bortezomib, and lenalidomide, was one of the major advances in frontline MM treatment during the past decade.2 For induction treatment, transplant-eligible patients usually receive several cycles of three-drug regimens (dexamethasone, bortezomib, and doxorubicin or cyclophosphamide or an immunomodulatory drug (IMiD)). Upon successful peripheral blood stem cell collection, high-dose melphalan therapy is given and autologous stem cell transplantation (ASCT) is performed, followed by lenalidomide maintenance therapy.3 Treatment choice at relapse is guided by clinical variables including performance status and age, as well as previous therapeutic lines and time to relapse. At first relapse after IMiD-based induction, doublet therapy with Kd (carfilzomib, low-dose dexamethasone) or Vd (bortezomib, low-dose dexamethasone), or triplet therapy based on bortezomib are approved. At first relapse after bortezomib-based induction, Rd (lenalidomide, low-dose dexamethasone) or Rd-backbone triplets are available.3 Despite novel treatment options, MM remains incurable and new treatment options are needed. According to the revised international staging system (R-ISS) for prognosis, the 5-year OS is 82, 62 and 40% for R-ISS stage I, II and III, respectively.4
Several tumour-associated antigens and immune escape mechanisms have been identified in MM, meaning immunotherapy is a promising treatment option, particularly in cases of relapsed/refractory disease. Chimeric antigen receptor (CAR) T cell immunotherapy has evolved as a potential anti-cancer therapy5 that has shown promising results in early clinical trials and, following the high response rates achieved in patients with pretreated and chemotherapy-resistant chronic and acute leukaemia and lymphoma, the FDA approved the application of CAR T cells in refractory CD19+ acute lymphoblastic leukaemia and diffuse large B cell lymphoma.6–10 Currently, CAR T cell therapy is being investigated in additional haematological neoplasia such as relapsed/refractory MM, where it has shown high remission rates and prolongation of progression-free survival.6,11–14
CAR T cells in the treatment of MM
CAR T cells are genetically modified T cells that express a chimeric antigen receptor (CAR). As CAR T cells are generated from autologous T cells collected from patients by leukapheresis, they represent an individualised therapy concept.15 Similar to monoclonal antibodies (mAbs), CARs are directed against specific cell-surface antigens, which should ideally only be expressed on target (tumour) cells and not on healthy tissues, to limit toxicity.16 Unlike mAbs, CAR T cells are not only able to kill target cells but might also induce a long-lasting immune response against the target antigen due to their persistence in vivo, aided by prior lymphodepletion by chemotherapy and radiotherapy to create a CAR T cell niche.5,17–19
Anti-tumour activity of CAR T cells in early clinical trials
The use of CAR T cells in the treatment of MM is currently limited to a few antigens and early-phase clinical trials. The B cell maturation antigen (BCMA) is particularly suitable as a CAR T cell target in MM, because it is specifically expressed on cells of the B lineage, including plasma cells and myeloma cells.20 BCMA is a member of the tumour necrosis factor receptor superfamily and is bound by ligands such as B cell-activating factor of the tumour necrosis factor family (BAFF) and a proliferation-inducing ligand (APRIL), which, as their names imply, induce B cell activation and proliferation.21 CAR T cells that target BCMA are currently being investigated in phase I clinical trials, and the preliminary results achieved in relapsed/refractory MM with regard to safety and efficacy are promising (Table 1).
Table 1.
CAR T cells in multiple myeloma early-phase clinical trials
| Antigen/ Reference | Trial design | CAR construct/ vector | CAR T cell dose | Conditioning/ lymphodepletion | Patients reported | Safety/side effects | Anti-tumour activity |
|---|---|---|---|---|---|---|---|
| BCMA | |||||||
| Ali et al.,22 Brudno et al.23 National Cancer Institute, National Institutes of Health, Bethesda | Phase I dose-escalation study |
Costimulation: CD28 Vector: γ retroviral |
0.3–9 × 106 cells/kg body weight | Cyclophosphamide (3 × 300 mg/m2) and fludarabine (3 × 30 mg/m2) | r/r MM, n = 27 | CRS and prolonged cytopenias in patients treated on the 9 × 106 cells/kg dose level | Anti-tumour activity of BCMA-CAR T cells in poor-prognosis MM demonstrated |
|
University of Pennsylvania, Philadelphia |
Phase I dose-escalation study |
Costimulation: 4-1BB Vector: lentiviral |
DL 1: 1–5 × 108 cells DL 2: 1–5 × 107 cells DL 3: 1–5 × 108 cells (absolute number) |
DL 1: none DL 2/3: cyclophosphamide 1.5 g/m2 |
r/r MM, n = 21 |
CRS (n = 17), severe reversible neurotoxicity (n = 3) |
Promising in vivo CAR T cell expansion and clinical activity, even without lymphodepletion Depth of response correlates with degree of BCMA-CAR T cell expansion and CRS |
|
Multicentre |
Multicentre phase I dose-escalation study |
Costimulation: 4-1BB Vector: lentiviral |
50–1200 × 106 cells (absolute number) |
Cyclophosphamide (3 × 300 mg/m2) and fludarabine (3 × 30 mg/m2) | r/r MM, n = 21 | CRS (n = 17) | Promising efficacy (100% ORR) at dose levels above 50 × 106 cells |
| Smith et al.28 Memorial Sloan-Kettering Cancer Center, New York | Phase I dose-escalation study |
Costimulation: 4-1BB Vector: retroviral |
DL 1: mean 72 × 106 cells DL 2: mean 137 × 106 cells (absolute number) |
DL 1: cyclophosphamide (1 × 3 g/m2) DL 2: cyclophosphamide/ fludarabine (3 × 300/30 mg/m2) |
r/r MM, n = 6 | CRS grade 1–2 (n = 3) | Promising anti-tumour activity in highly pretreated patients |
|
Mi et al.,31 Fan et al.30 Shanghai Institute of Hematology, Shanghai Jiao Tong University, Shanghai |
Phase I |
Antigen recognition: bi-epitope Costimulation: not published vector: not published (LCAR-B38M) |
Median 4.7 × 106 cells/kg BW infused over 3 days | Cyclophosphamide (3 × 250 mg/m2) and fludarabine (3 × 25 mg/m2) | r/r MM, n = 19 | CRS (n = 14) | Objective response achieved in all patients, CR/nCR in 19 patients |
| CD19 | |||||||
| Garfall et al.33, 34 University of Pennsylvania, Philadelphia | Phase I |
Costimulation: 4-1BB Vector: lentiviral (CTL019) |
1–5 × 107 cells (absolute number) |
Melphalan (140–200 mg/m2) and ASCT | r/r MM, n = 10 | Most toxicity attributable to ASCT, no severe CRS | CTL019 may prolong response of standard MM therapies |
| CD138 | |||||||
|
Guo et al.38 General Hospital of PLA, Beijing |
Phase I |
Costimulation: 4-1BB Vector: lentiviral |
0.756 × 107 cells/kg BW | CP or PCD or VAD | r/r MM, n = 5 | No intolerable toxicities, grade 3 fever upon CAR T cell infusion | Feasibility demonstrated, stable disease in four patients longer than 3 months |
| κ-light-chain | |||||||
|
Ramos et al.39 Baylor College of Medicine, Houston |
Phase I |
Costimulation: CD28 Vector: retroviral |
0.2–2 × 108 cells/m2 BS | Cyclophosphamide (12.5 mg/kg) in patients without lymphopenia | r/r MM, n = 7 | No toxicities attributable to CAR T cells | Stable disease 4 patients lasting 2–17 months |
| NKG2D ligands | |||||||
|
Nikiforow et al.43 Dana-Farber Cancer Institute, Boston |
Phase I dose-escalation study |
Receptor design: NKG2D, DAP10 signal transmission subunit, CD3ζ Signalling domain vector: retroviral (CM-CS1) |
1 × 106–3 × 107 cells (absolute number) |
None | r/r MM, n = 5 | Safety demonstrated, no dose-limiting toxicity | Feasibility demonstrated |
ASCT autologous stem cell transplantation, BCMA B cell maturation antigen, BW body weight, BS body surface, CAR chimeric antigen receptor, CRS cytokine-release syndrome, DL dose level, MM multiple myeloma, (n)CR (near) complete response, ORR overall response rate, r/r relapsed/refractory
Literature research was mainly based on the ASH annual meeting abstracts considering the search terms “CAR/chimeric antigen receptor and multiple myeloma” from all years (number of screened abstracts >300). The table makes no claim to be comprehensive
Ali et al.22 and Brudno et al.23 published the first results of a phase I dose-escalation trial of BCMA-CAR T cell treatment (0.3–9 × 106 CAR T cells/kg body weight) in 27 patients with relapsed/refractory MM, in which the anti-tumour activity of BCMA-targeted CAR T cells in poor-prognosis MM was demonstrated, using a cyclophosphamide/fludarabine conditioning regimen. Cytokine-release syndrome (CRS) and prolonged cytopenia occurred in patients treated with the 9 × 106 CAR T cells/kg dose.22,23 Cohen et al.24 carried out a phase I dose-escalation study using a fully human BCMA-specific CAR with CD3ζ and 4-1BB signalling domains, the results of which showed promising in vivo CAR T cell expansion and clinical activity in 21 highly pretreated MM patients, even without lymphodepletion. CRS, characterised by increased levels of circulating cytokines such as interleukin-6 (IL-6), was reported in 17 patients (six of whom showed CRS grade 3–4) and severe reversible neurotoxicity was reported in three patients. Interestingly, the depth of response correlated with the degree of BCMA-CAR T cell expansion and CRS.25 In a separate study, Berdeja et al.26,27 treated 21 relapsed/refractory MM patients in a multicentre phase I dose-escalation trial with a second-generation BCMA-targeted CAR T cell construct upon lymphodepletion with fludarabine and cyclophosphamide, and reported manageable CRS, no dose-limiting toxicities, and promising anti-MM efficacy at dose levels above 50 × 106 CAR T cells, achieving an overall response rate (ORR) of 100%. Similarly, Smith et al.28,29 reported promising results in a small cohort of six patients with relapsed/refractory MM treated with BCMA-CAR T cells. Using a technique known as bi-epitope targeting, Fan et al.30 and Mi et al.31 reported on the clinical application of CAR T cells engineered to target two distinct regions of BCMA in a cohort of 19 relapsed/refractory MM patients. CRS was reported in 14 patients and was manageable. Of particular interest, a 100% ORR was achieved and 18 of the patients (95%) reached complete remission or near-complete remission. No relapses were observed at a median follow-up of 6 months.30,31
Although usually expressed on B cells, the B cell co-receptor CD19 can also be found on a small proportion of myeloma cells that might represent MM cancer stem cells.15 In a 2014 phase I clinical trial of 10 patients with relapsed/refractory MM,32 CD19-CAR T cells were administered approximately 2 weeks after treatment with high-dose melphalan and autologous stem cell transplant (ASCT). The CAR construct included an anti-CD19 single-chain variable fragment linked to the 4-1BB and CD3ζ signalling domains.7 No severe CRS was observed, and most of the reported toxicity was attributable to the ASCT. Two patients showed significantly longer progression-free survival after CD19-targeted CAR T cell therapy was incorporated into the strategy, compared with prior high-dose melphalan and ASCT alone, prompting the authors to emphasise the possible additional use of CD19-CAR T cells in order to prolong the duration of response to standard myeloma treatment.33,34 Interestingly, CD19 expression on the myeloma cells was very low. Due to the inconsistent or absent expression of CD19 in the majority of patients with MM,35 the mechanism of action of CD19-targeted CAR T cells is controversial. Possible explanations for the positive results include the presence of a small population of CD19+ myeloma precursor cells, very low and undetectable CD19 expression on myeloma cells, and/or the eradication of non-malignant CD19+ B cells that might otherwise suppress the anti-tumour immune response.36
CD138 is a member of the syndecan family that is involved in cell–cell and cell–matrix interactions and is predominantly expressed on the surface of epithelial cells, plasma cells, and myeloma cells.37 Guo et al.38 designed a phase I clinical trial for relapsed/refractory MM patients using CAR T cells that target CD138. Results obtained from the five patients enrolled on this trial seem to be promising: four of the patients achieved stable disease for at least 3 months (range 3–7 months), whereas the fifth patient, whose MM had progressed to plasma cell leukaemia, showed a reduction in the level of myeloma cells in the peripheral blood. No relevant toxicities, particularly no epithelial damage, were observed.
Ramos et al.39 have constructed CAR T cells specific for the κ-light chain in order to target malignant cells in which κ-light-chain expression is restricted (e.g. in B cell and plasma cell malignancies) and to spare normal B cells expressing the non-targeted λ-light chain. In a phase I clinical trial including five patients with MM, the investigators reported that four of the patients achieved stable disease lasting 2–17 months.39
Natural killer group 2, member D (NKG2D) is an activating receptor usually expressed on the surface of immune cells, particularly on natural killer (NK) cells, T cells (γδ, and CD8+ and CD4+ subsets) and invariant NKT cells. NKG2D promotes the elimination of NKG2D-ligand expressing cells. On NK cells NKG2D serves as an activating receptor that triggers cytotoxicity upon ligand binding. In CD8+ T cells NKG2D promotes co-stimulatory signals.40 While absent in healthy tissues, the expression of NKG2D ligands (MIC-A, MIC-B and various UL16-binding proteins/RAET1 proteins) is upregulated in infected cells and various cancer types.41 T cells expressing an NKG2D-CAR construct induced significant anti-tumour activity in murine tumour models.42 Nikiforow et al.43 have demonstrated the safety and feasibility of a single infusion of NKG2D-targeted CAR T cells in five patients with relapsed/refractory MM in the first three dose-escalation cohorts in a phase I dose-escalation clinical study.
Additional antigens investigated in preclinical CAR T cell studies
Further target antigens for MM CAR T cell therapies have been explored over the past few years, with recent studies particularly focussed on antigens such as signalling lymphocytic activation molecule F7 (SLAMF7; also known as CS1 and CD319) and CD38.
SLAMF7 is highly expressed in normal and neoplastic plasma cells and different immune cells (B, NK, NKT, T cells, dendritic cells and monocytes), but not in normal tissue parenchyma.44–47 Elotuzumab is a SLAMF7-directed mAb, which is currently under clinical investigation in newly diagnosed and relapsed or refractory MM.48–50 CAR-engineered cells specific for SLAMF7 are currently under development as both autologous and allogeneic ‘off-the-shelf’ approaches. Danhof et al.51 and Gogishvili et al.52 designed a SLAMF7-CAR construct derived from elotuzumab. SLAMF7-CAR T cells derived from MM patients and healthy donors demonstrated effective in vitro cytolysis of primary myeloma cells. Furthermore, in a xenograft mouse model SLAMF7-CAR T cells showed promising in vivo activity by significant reduction of medullary and extramedullary myeloma manifestations. Importantly, the SLAMF7-CAR T cells not only recognised MM cells but also selectively targeted SLAMF7+/-high B, NK, NKT and T cells, while sparing SLAMF7-negative/-low immune cells. The authors hypothesised that lymphocytic fratricide by SLAMF7-CAR T cells might lead to acute and chronic side effects such as cytokine storm and viral infections, when used in a clinical setting.51,52 Wang et al.53 also reported on a SLAMF7-CAR encoded by a lentiviral vector and containing a CD28 co-stimulatory domain. Two mutations on the IgG4 linker CH-2 portion were introduced to enhance the potency and persistence of the SLAMF7-CAR T cells. In vitro cell culture analysis revealed specific lysis of MM cell lines by SLAMF7-CAR T cells. In addition, compared to CARs directed against other antigens overexpressed by MM (BCMA and CD44v6 (CD44 adhesion receptor isoform variant 6), SLAMF7-CAR T cell administration resulted in the best anti-tumour activity in an MM-bearing mouse model.
These promising results support the suitability of SLAMF7-CARs for further evaluation in early-phase clinical trials.53 Furthermore, in terms of a potential universal ‘off-the-shelf’ approach, Galetto et al.54 and Mathur et al.55 designed allogeneic SLAMF7-CAR T cells derived from healthy donor peripheral blood mononuclear cells (PBMCs). As TCR-deficient T cells were demonstrated not to mediate alloreactivity in a xenograft-versus-host disease (GvHD) mouse model, the TCRα constant (TRAC) gene was inactivated to reduce the GvHD potential of the allogeneic SLAMF7-CAR T cells. Furthermore, the researchers aimed to minimise the risk of fratricide by SLAMF7 gene inactivation in SLAMF7-CAR T cells. The allogeneic SLAMF7-CAR T cells specifically lysed MM cell lines and primary MM tumour cells. In an MM mouse model, a single injection of 10 × 106 SLAMF7-CAR T cells resulted in a substantial decrease of serum monoclonal protein levels. Designing double-knockout (TCRA and SLAMF7) allogeneic SLAMF7-CAR T cells, the authors demonstrated the feasibility of the multiplex genome editing CAR approach, making the first steps towards the large-scale availability of SLAMF7-CAR T cells to MM patients.55,54
CD38 is frequently expressed on normal and aberrant plasma cells,56–58 and recent immunotherapeutic approaches have targeted CD38 with mAbs, including daratumumab, isatuximab, MOR03087, and Ab79.59–63 CD38 is not only found in plasma cells but also in lower levels on other immune cells such as B cells, B cell progenitors, NK cells, monocytes and hematopoietic cells.64 Drent et al.65,66 reported on CD38-CAR T cells based on three different CD38 antibodies, CD3ζ and 4-1BB signalling domains. Derived from healthy donor PBMCs, CD38-T cell CARs effectively lysed MM cell lines and primary MM cells. Significant in vivo anti-tumour effect was also observed in a xenograft mouse model. Furthermore, first steps towards safe clinical use were made by transduction of CD38-CAR T cells with a caspase-9-based inducible suicide gene that allowed effective control of CD38-CAR T cells. However, targeting MM cells with high-affinity CD38-CAR T cells also led to on-target, off-tumour effects, reflected by the lysis of normal CD38+ hematopoietic cells. To overcome this drawback, the authors used a ‘light-chain exchange method’, to produce CD38-CARs with up to 1000-fold lower affinity to CD38. Those low-affinity CD38-CAR T cells still effectively lysed CD38+ MM cells while little activity was observed against CD38+ hematopoietic cells in vitro and in vivo. Through this, the authors successfully demonstrated that optimising CAR affinity is feasible, and may improve safety in clinical applications.65,66
CD44v6 was shown to be expressed in epithelial and haematologic tumours, including advanced high-risk MM.67 CD44v6 is also expressed on keratinocytes and immune cells as monocytes and T cells at low levels, and almost no expression is found on CD34+ haematopoietic stem cells.68 Casucci et al.69,70 aimed to target CD44v6 by a CAR construct containing a single-chain variable fragment (scFv) of humanised CD44v6 mAb, CD28 co-stimulatory domain and inducible caspase-9 suicide gene. CD44v6-CAR T cells showed long-term persistence and eradication of previously engrafted MM tumour cells in an in vivo mouse model, and no cytotoxic activity was observed against hematopoietic stem cells. The authors are currently designing a phase I/II trial of CD44v6-CAR T cell administration after ASCT.
CD229 has been demonstrated to be strongly expressed on MM cells and MM precursors, predisposing it as a potential therapeutic target.71,72 Radhakrishnan et al.73 generated the first humanised CD229 scFv, to create a CAR construct using a 4-1BB co-stimulatory domain. CD229-CAR T cells had strong cytotoxic activity against CD229+ MM cell lines and completely eradicated MM cells in a mouse model after only 18 days. Only limited toxicity was observed against other immune cells such as B and resting T cells. The authors plan to evaluate the efficacy of CD229-CAR T cells in relapsed/refractory MM patients in early-phase clinical trials.73
NK cells for CAR-based immunotherapy in MM
Beyond the CAR T cell approach, recent studies have aimed to engineer NK cells using CAR technology, in order to increase their reactivity and recognition specificity towards myeloma cells. NK cells are effector lymphocytes of the innate immune system. NK cell cytotoxicity is regulated by signals from stimulatory (e.g. FcγRIII-mediated antibody-dependent cell-mediated cytotoxicity) and inhibitory receptors (e.g. killer-cell immunoglobulin-like receptors during downregulation of major histocompatibility class I (MHC I) on target cells). NK cells mediate cytotoxicity via perforin and granzyme release and the expression of apoptosis-inducing ligands (e.g., FasL and TRAIL).74 CAR NK cells could have several advantages over CAR T cells, including a presumed better safety profile, as well as a potentially higher anti-tumour activity due to their multiple activation modalities. Mature NK cells are short-lived cells, meaning no long-lasting toxicities are expected in NK cell-based CAR approaches, and suicide genes might not be required. Furthermore, although CAR expression would direct NK cells towards malignant cells, NK cell cytotoxicity can also be triggered in a CAR-independent manner via stimulatory and inhibitory receptors, presumably increasing the potential for anti-tumour activity.74–76
The generation of CAR NK cells directed against MM antigens and their in vitro (cell lines)/ex vivo (primary patient samples) anti-tumour activity has been demonstrated in several preclinical studies. Chu et al.77 successfully generated SLAMF7-targeted CAR NK cells that displayed MM cytolytic activity in vitro and ex vivo, as well as efficient suppression of tumour growth in an aggressive orthotopic MM xenograft mouse model.77 A CAR NK cell approach to target CD138 on MM cells was used by Jiang et al.78 who generated NK cells carrying a CAR consisting of an anti-CD138 scFv and CD3ζ. Furthermore, Leivas et al.79 produced NKG2D-targeted CAR NK cells by transducing autologous-activated and -expanded NK cells with NKG2D-CAR-containing 4-1BB and CD3ζ signalling domains. NKG2D-CAR NK cells showed significant cytotoxic activity against MM cells in vitro. These encouraging results warrant further development of CAR NK cells in preclinical and early-phase clinical trials for MM treatment.
Genetically modified TCR approaches
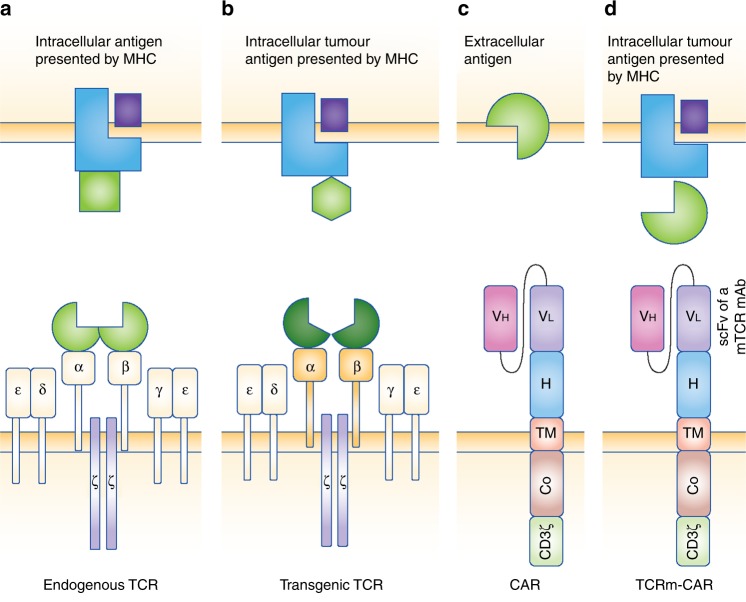
CARs provide a highly specific mechanism to target tumour antigens in an MHC-independent manner; however, they are limited to extracellular antigens. Addressing intracellular therapeutically relevant antigens might be advantageous in order to increase the number of tumour-derived targets, and cellular therapies based on engineered (transgenic) TCRs have been developed.80 Contrary to CARs, TCRs can recognise intracellular antigens presented by the MHC, and genetic modifications of TCRαβ sequences can be applied in order to change the affinity of the TCR, redirecting it towards therapeutically relevant antigens (Fig. 1). 81 Going one step further, TCR-mimic (TCRm) antibody CAR T cells were developed. These CAR constructs are similar to the usual CARs, but they are derived from antibodies that mimic TCR function by recognising peptides presented on MHC I (the so-called TCRm or TCR-like antibodies). TCRm-CAR T cells are therefore able to recognise intracellular antigens presented by human leucocyte antigen (HLA) haplotypes.82,83
Fig. 1.
Principle structure of endogenous, engineered T cell receptor (TCR), chimeric antigen receptor (CAR) and TCR-mimic CAR. Endogenous and transgenic TCRs recognise intracellular peptides that are presented by the major histocompatibility complex (MHC). Additional co-stimulatory signals are required for complete T cell activation. a Endogenous TCRs consist of paired α and β chains (antigen recognition in context of MHC) associated with δ, ε, γ, and signalling ζ chains. b Transgenic TCR TCRαβ chains are genetically engineered to enhance or modify affinity. c Chimeric antigen receptors (CARs) recognise extracellular antigens independent of the MHC. The extracellular portion of the CAR consists of single-chain variable fragment (scFv) of a monoclonal antibody (heavy- and light-chain variable domains—VH/VL-specific for the targeted surface antigen) and a hinge region (H, stabilisation). The transmembrane domain (TM) serves as an anchor to the cell membrane. One or more intracellular co-stimulatory (Co, e.g. CD27, CD28, 4-1BB, OX40) and a CD3ζ chain domain represent signal transduction domains. d TCR-mimic antibody- (TCRm-) CARs are similar to the usual CAR constructs. Derived from monoclonal antibodies that mimic TCR function (TCRm mAb), TCRm-CARs thus recognise intracellular peptides presented on MHC I. Figure adopted from Fesnak et al.81
Recent studies have demonstrated the feasibility and therapeutic efficacy of engineered TCR T cell approaches in malignant tumours.84 Furthermore, transgenic TCR T cell therapies are being investigated as an MM treatment, with current research focussing on targeting cancer testis antigens such as NY-ESO1, the B cell-specific transcription factor BOB1, or the intracellular transcription factor Wilms tumour-1 (WT1).
Several groups have developed engineered TCR and mTCR-CAR approaches to target NY-ESO-1, which was found to be highly expressed in poor-prognosis MM.85–90 Maruta et al.85 developed an mTCR-CAR T cell specific for an NY-ESO-1 that was presented by a HLA-A*02:01 molecule, and which showed significant anti-MM reactivity. Patel et al.86,87 demonstrated that NY-ESO-1 CAR T cell efficacy can be augmented by an NY-ESO-1+ T antigen-presenting cell (T-APC) vaccine. Superior anti-MM activity was observed in an MM mouse model when treated with both NY-ESO-1-CAR T cells and a T-APC vaccine, compared with CAR T cell treatment alone. Finally, clinical feasibility has been investigated by Rapoport et al.89 via a phase I/II clinical trial that focussed on targeting a peptide of NY-ESO-1 and of LAGE-1, which shows high homology to NY-ESO-1. Twenty antigen-positive MM patients with advanced disease were included and received autologous modified TCR T cells with enhanced affinity towards NY-ESO-1 and LAGE-1, following high-dose melphalan and ASCT. Upon a median follow-up of over 20 months, 14 (70%), 2 (10%) and 2 (10%) patients reached near/complete response, very good partial response and partial response, respectively. One patient (5%) had stable disease and 1 (5%) patient progressed. Overall, the authors demonstrated feasibility, safety, and promising anti-MM activity of NY-ESO-1-LAGE-1 TCR-engineered T cells.89
Aiming to target the intracellular B cell-specific transcription factor BOB1, Jan et al.91–93 isolated and transduced a TCR specifically recognising a peptide of BOB1 in the context of HLA-B*07:02 to recipient T cells. The transduced T cells efficiently lysed primary tumour cells of different haematological malignancies, including MM, and revealed potent anti-tumour response in an in vivo MM xenograft mouse model. As B cells also show BOB1 expression, significant B cell lysis was detected.91–93
Rafiq et al.93 designed a TCRm-CAR T cell that was directed against WT1, a transcription factor that was shown to be overexpressed in leukaemias and several solid tumours. In MM, WT1 was demonstrated to serve as an additional marker for risk stratification.94 The TCRm-CAR construct was derived from a monoclonal TCRm antibody (WT1 28z) recognising a specific peptide of the WT1 protein in the context of HLA-A*02:01. WT1 28z-CAR T cells showed WT1-HLA-A*02:01-specific cytotoxicity against various cancer cell lines, including MM. The authors aim to transfer this new adoptive mTCR-CAR T cell approach to the clinical setting for haematological and solid tumours.95
Conclusions
Despite the advent of novel therapies and improved outcomes, patients with relapsed/refractory MM have a poor prognosis, and MM is usually an incurable disease. Improved treatment strategies are therefore needed for this malignancy. CAR T cell therapy appears to be a promising treatment option for MM. So far, the clinical use of CAR T cells is limited to a few antigens, early-phase trials, and patients with relapsed/refractory disease. Promising results with high response rates and manageable toxicities have been obtained in phase I/II trials using CAR T cells that target BCMA, CD19, CD138 or κ-light chain. Furthermore, preclinical studies on CD38-CAR T cells and SLAMF7-CAR T cells in the treatment of MM have yielded encouraging results that merit further investigation. Owing to these promising results, it is possible that CAR T cells will become a standard treatment option for relapsed/refractory MM over the next few years. Further investigation is needed in several areas, including identification of new targets, optimisation of CAR design to improve efficacy, and the combination of this treatment with other therapeutic approaches.
Acknowledgements
Author contributions
H.G., M.S. and P.D. conceived of the presented idea. K.K., M.K. and M.H. wrote the manuscript. M.C. and C.M.-T. aided in manuscript concept. All authors reviewed the final manuscript.
Consent for publication
The consent for publication is given by all authors.
Competing interests
The authors declare no competing interests.
References
- 1.Palumbo A, et al. Personalized therapy in multiple myeloma according to patient age and vulnerability: a report of the European Myeloma Network (EMN) Blood. 2011;118:4519–4529. doi: 10.1182/blood-2011-06-358812. [DOI] [PubMed] [Google Scholar]
- 2.Moreau P, Attal M, Facon T. Frontline therapy of multiple myeloma. Blood. 2015;125:3076–3084. doi: 10.1182/blood-2014-09-568915. [DOI] [PubMed] [Google Scholar]
- 3.Moreau P, et al. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017;28(Suppl. 4):iv52–iv61. doi: 10.1093/annonc/mdx096. [DOI] [PubMed] [Google Scholar]
- 4.Palumbo A, et al. Revised international staging system for multiple myeloma: a report from International Myeloma Working Group. J. Clin. Oncol. 2015;33:2863–2869. doi: 10.1200/JCO.2015.61.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 6.Hudecek M, Einsele H. Myeloma CARs are rolling into the clinical arena. Blood. 2016;128:1667–1668. doi: 10.1182/blood-2016-08-729467. [DOI] [PubMed] [Google Scholar]
- 7.Maude SL, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grupp SA, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salter, A. I., Pont, M. J. & Riddell, S. R. Chimeric antigen receptor modified T cells: CD19 and the road beyond. Blood. 131, 2621–2629. (2018). [DOI] [PMC free article] [PubMed]
- 11.Nagle SJ, Garfall AL, Stadtmauer EA. The promise of chimeric antigen receptor engineered T cells in the treatment of hematologic malignancies. Cancer J. 2016;22:27–33. doi: 10.1097/PPO.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sohail A, et al. Emerging immune targets for the treatment of multiple myeloma. Immunotherapy. 2018;10:265–282. doi: 10.2217/imt-2017-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CAR T cell therapy impresses in multiple myeloma. Cancer Discov. 8: OF2 (2018). 10.1158/2159-8290 [DOI] [PubMed]
- 14.Prommersberger S, et al. Novel targets and technologies for CAR-T cells in multiple myeloma and acute myeloid leukemia. Curr. Res. Transl. Med. 2018;66:37–38. doi: 10.1016/j.retram.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Mikkilineni L, Kochenderfer JN. Chimeric antigen receptor T cell therapies for multiple myeloma. Blood. 2017;130:2594–2602. doi: 10.1182/blood-2017-06-793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan RA, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brentjens RJ, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat. Rev. Immunol. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gattinoni L, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J. Exp. Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carpenter RO, et al. B-cell maturation antigen is a promising target for adoptive T cell therapy of multiple myeloma. Cancer Res. 2013;19:2048–2060. doi: 10.1158/1078-0432.CCR-12-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mackay F, Schneider P, Rennert P, Browning J, BAFF AND. APRIL: a tutorial on B cell survival. Annu. Rev. Immunol. 2003;21:231–264. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 22.Ali SA, et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128:1688–1700. doi: 10.1182/blood-2016-04-711903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brudno J, et al. T cells genetically modified to express an anti-B-cell maturation antigen chimeric antigen receptor with a CD28 costimulatory moiety cause remissions of poor-prognosis relapsed multiple myeloma. Blood. 2017;130(Suppl. 1):524. doi: 10.1200/JCO.2018.77.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen AD, et al. B-cell maturation antigen (BCMA)-specific chimeric antigen receptor T cells (CART-BCMA) for multiple myeloma (MM): initial safety and efficacy from a phase I study. Blood. 2016;128:1147. [Google Scholar]
- 25.Cohen AD, et al. Safety and efficacy of B-cell maturation antigen (BCMA)-specific chimeric antigen receptor T cells (CART-BCMA) with cyclophosphamide conditioning for refractory multiple myeloma (MM) Blood. 2017;130(Suppl. 1):505. [Google Scholar]
- 26.Berdeja JG, et al. First-in-human multicenter study of bb2121 anti-BCMA CAR T cell therapy for relapsed/refractory multiple myeloma: updated results. J. Clin. Oncol. 2017;35(Suppl.):3010. doi: 10.1200/JCO.2017.35.15_suppl.3010. [DOI] [Google Scholar]
- 27.Berdeja JG, et al. Durable clinical responses in heavily pretreated patients with relapsed/refractory multiple myeloma: updated results from a multicenter study of bb2121 anti-BCMA CAR T cell therapy. Blood. 2017;130(Suppl. 1):740. [Google Scholar]
- 28.Smith EL, et al. Development and evaluation of a human single chain variable fragment (scFv) derived BCMA targeted CAR T cell vector leads to a high objective response rate in patients with advanced MM. Blood. 2017;130(Suppl. 1):742. [Google Scholar]
- 29.Smith EL, et al. Development and evaluation of an optimal human single-chain variable fragment-derived BCMA-targeted CAR T cell vector. Mol. Ther. 2018;26:1447–1456. doi: 10.1016/j.ymthe.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan F, et al. Durable remissions with BCMA-specific chimeric antigen receptor (CAR)-modified T cells in patients with refractory/relapsed multiple myeloma. J. Clin. Oncol. 2017;35(Suppl):LBA3001–LBA3001. doi: 10.1200/JCO.2017.35.15_suppl.LBA3001. [DOI] [Google Scholar]
- 31.Mi JQ, et al. Effective treatment of relapsed/refractory multiple myeloma including extramedullary involvement by BCMA-specific chimeric antigen receptor-modified T cells. Blood. 2017;130(Suppl. 1):3115. [Google Scholar]
- 32.ClinicalTrials.gov. CART-19 for Multiple Myeloma, NCT021354062014 (updated 16.03.2017). Available from: https://clinicaltrials.gov/ct2/show/record/NCT02135406
- 33.Garfall AL, et al. Chimeric antigen receptor T Ccells against CD19 for multiple myeloma. N. Engl. J. Med. 2015;373:1040–1047. doi: 10.1056/NEJMoa1504542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garfall, A. L. et al. Anti-CD19 CAR T cells with high-dose melphalan and autologous stem cell transplantation for refractory multiple myeloma. JCI Insight3, pii: 120505. 10.1172/jci.insight.120505. (2018). [DOI] [PMC free article] [PubMed]
- 35.Tembhare PR, et al. Flow cytometric differentiation of abnormal and normal plasma cells in the bone marrow in patients with multiple myeloma and its precursor diseases. Leuk. Res. 2014;38:371–376. doi: 10.1016/j.leukres.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimabukuro-Vornhagen A, Schloesser H, Bergwelt-Baildon MS. Chimeric antigen receptor T cells in myeloma. N. Engl. J. Med. 2016;374:193–194. doi: 10.1056/NEJMc1512760. [DOI] [PubMed] [Google Scholar]
- 37.Palaiologou M, Delladetsima I, Tiniakos D. CD138 (syndecan-1) expression in health and disease. Histol. Histopathol. 2014;29:177–189. doi: 10.14670/HH-29.177. [DOI] [PubMed] [Google Scholar]
- 38.Guo B, et al. CD138-directed adoptive immunotherapy of chimeric antigen receptor (CAR)-modified T cells for multiple myeloma. J. Cell Immunother. 2016;2:28–35. doi: 10.1016/j.jocit.2014.11.001. [DOI] [Google Scholar]
- 39.Ramos CA, et al. Clinical responses with T lymphocytes targeting malignancy-associated kappa light chains. J. Clin. Invest. 2016;126:2588–2596. doi: 10.1172/JCI86000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jamieson AM, et al. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity. 2002;17:19–29. doi: 10.1016/S1074-7613(02)00333-3. [DOI] [PubMed] [Google Scholar]
- 41.Lanier LL. NKG2D receptor and its ligands in host defense. Cancer Immunol. Res. 2015;3:575–582. doi: 10.1158/2326-6066.CIR-15-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spear P, Barber A, Rynda-Apple A, Sentman CL. NKG2D CAR T cell therapy inhibits the growth of NKG2D ligand heterogeneous tumors. Immunol. Cell Biol. 2013;91:435–440. doi: 10.1038/icb.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nikiforow S, et al. Safety data from a first-in-human phase 1 trial of NKG2D chimeric antigen receptor-T cells in AML/MDS and multiple myeloma. Blood. 2016;128:4052. [Google Scholar]
- 44.Hsi ED, et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin. Cancer Res. 2008;14:2775–2784. doi: 10.1158/1078-0432.CCR-07-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee JK, Mathew SO, Vaidya SV, Kumaresan PR, Mathew PA. CS1 (CRACC, CD319) induces proliferation and autocrine cytokine expression on human B lymphocytes. J. Immunol. 2007;179:4672–4678. doi: 10.4049/jimmunol.179.7.4672. [DOI] [PubMed] [Google Scholar]
- 46.Frigyesi I, et al. Robust isolation of malignant plasma cells in multiple myeloma. Blood. 2014;123:1336–1340. doi: 10.1182/blood-2013-09-529800. [DOI] [PubMed] [Google Scholar]
- 47.Malaer JD, Mathew PA. CS1 (SLAMF7, CD319) is an effective immunotherapeutic target for multiple myeloma. Am. J. Cancer Res. 2017;7:1637–1641. [PMC free article] [PubMed] [Google Scholar]
- 48.Richardson, P. G., Jagannath, S. & Moreau, P. Final results for the 1703 phase 1B/2 study of elotuzumab in combination with lenalidomide and dexamethasone in patients with relapsed/refractory multiple myeloma. In 56th ASH Annual Meeting and ExpositionLancet Haematol. 2, e516–27. (2015). [DOI] [PMC free article] [PubMed]
- 49.Usmani, S. Z., Sexton, R. & Ailawadhi,S. Initial report on PHASE I Trial of RVD-elotuzumab for newly diagnosed high risk multiple myeloma (HRMM). In 56th ASH Annual Meeting and ExpositionLancet Haematol. Leukemia, 30, 526–535 (2015).
- 50.Lonial S, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N. Engl. J. Med. 2015;373:621–631. doi: 10.1056/NEJMoa1505654. [DOI] [PubMed] [Google Scholar]
- 51.Gogishvili T, et al. SLAMF7-CAR T cells eliminate myeloma and confer selective fratricide of SLAMF7+ normal lymphocytes. Blood. 2017;130:2838–2847. doi: 10.1182/blood-2017-04-778423. [DOI] [PubMed] [Google Scholar]
- 52.Danhof S, et al. CAR-engineered T cells specific for the elotuzumab target SLAMF7 eliminate primary myeloma cells and confer selective fratricide of SLAMF7+ normal lymphocyte subsets. Blood. 2015;126:115. doi: 10.1182/blood-2017-04-778423. [DOI] [PubMed] [Google Scholar]
- 53.Wang X, et al. CS-1 re-directed central memory T cell therapy for multiple myeloma. Blood. 2014;124:1114. [Google Scholar]
- 54.Galetto R, Chion-Sotinel I, Gouble A, Smith J. Bypassing the constraint for chimeric antigen receptor (CAR) development in T cells expressing the targeted antigen: improvement of anti-CS1 CAR activity in allogenic TCRa/CS1 double knockout T cells for the treatment of multiple myeloma (MM) Blood. 2015;126:116. [Google Scholar]
- 55.Mathur R, et al. Universal SLAMF7-specific CAR T cells as treatment for multiple myeloma. Blood. 2017;130(Suppl. 1):502. [Google Scholar]
- 56.Flores-Montero J, et al. Immunophenotype of normal vs. myeloma plasma cells: toward antibody panel specifications for MRD detection in multiple myeloma. Cytom. B. 2016;90:61–72. doi: 10.1002/cyto.b.21265. [DOI] [PubMed] [Google Scholar]
- 57.Perez-Andres M, et al. Human peripheral blood B-cell compartments: a crossroad in B-cell traffic. Cytom. B. 2010;78(Suppl. 1):S47–S60. doi: 10.1002/cyto.b.20547. [DOI] [PubMed] [Google Scholar]
- 58.Terstappen LW, Johnsen S, Segers-Nolten IM, Loken MR. Identification and characterization of plasma cells in normal human bone marrow by high-resolution flow cytometry. Blood. 1990;76:1739–1747. [PubMed] [Google Scholar]
- 59.Kumar S, Kimlinger T, Morice W. Immunophenotyping in multiple myeloma and related plasma cell disorders. Best. Pract. Res. Clin. Haematol. 2010;23:433–451. doi: 10.1016/j.beha.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lammerts van Bueren J, et al. Direct in vitro comparison of daratumumab with surrogate analogs of CD38 antibodies MOR03087, SAR650984 and Ab79. Blood. 2014;124:3474. [Google Scholar]
- 61.Lokhorst HM, et al. Dose-dependent efficacy of daratumumab (DARA) as monotherapy in patients with relapsed or refractory multiple myeloma (RR MM) J. Clin. Oncol. 2014;32(Suppl.):8513. doi: 10.1200/jco.2014.32.15_suppl.8513. [DOI] [Google Scholar]
- 62.van de Donk NW, et al. Monoclonal antibodies targeting CD38 in hematological malignancies and beyond. Immunol. Rev. 2016;270:95–112. doi: 10.1111/imr.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shallis, R. M., Terry, C. M. & Lim S. H. The multi-faceted potential of CD38 antibody targeting in multiple myeloma. Cancer Immunol. Immunother.66, 697–703. (2017). [DOI] [PMC free article] [PubMed]
- 64.Quarona V, et al. CD38 and CD157: a long journey from activation markers to multifunctional molecules. Cytom. Part B. 2013;84:207–217. doi: 10.1002/cyto.b.21092. [DOI] [PubMed] [Google Scholar]
- 65.Drent E, et al. Reducing on-target off-tumor effects of CD38-chimeric antigen receptors by affinity optimization. Blood. 2016;128:2170. doi: 10.1016/j.ymthe.2017.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Drent E, et al. CD38 chimeric antigen receptor engineered T cells as therapeutic tools for multiple myeloma. Blood. 2014;124:4759. [Google Scholar]
- 67.Liebisch P, et al. CD44v6, a target for novel antibody treatment approaches, is frequently expressed in multiple myeloma and associated with deletion of chromosome arm 13q. Haematologica. 2005;90:489–493. [PubMed] [Google Scholar]
- 68.Neu S, et al. Expression of CD44 isoforms by highly enriched CD34-positive cells in cord blood, bone marrow and leukaphereses. Bone Marrow Transplant. 1997;20:593–598. doi: 10.1038/sj.bmt.1700940. [DOI] [PubMed] [Google Scholar]
- 69.Casucci M, et al. Co-expression of a suicide gene in CAR-redirected T cells enables the safe targeting of CD44v6 for leukemia and myeloma eradication. Blood. 2012;120:949. [Google Scholar]
- 70.Casucci M, et al. CD44v6-targeted T cells mediate potent antitumor effects against acute myeloid leukemia and multiple myeloma. Blood. 2013;122:3461–3472. doi: 10.1182/blood-2013-04-493361. [DOI] [PubMed] [Google Scholar]
- 71.Yousef S, et al. CD229 is expressed on the surface of plasma cells carrying an aberrant phenotype and chemotherapy-resistant precursor cells in multiple myeloma. Hum. Vaccines Immunother. 2015;11:1606–1611. doi: 10.1080/21645515.2015.1046658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Atanackovic D, et al. Surface molecule CD229 as a novel target for the diagnosis and treatment of multiple myeloma. Haematologica. 2011;96:1512–1520. doi: 10.3324/haematol.2010.036814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Venniyil Radhakrishnan S, et al. Chimeric antigen receptor (CAR) T cells specific for CD229: a potentially curative approach for multiple myeloma. Blood. 2017;130(Suppl. 1):3142. [Google Scholar]
- 74.Smyth MJ, et al. Activation of NK cell cytotoxicity. Mol. Immunol. 2005;42:501–510. doi: 10.1016/j.molimm.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 75.Klingemann H. Are natural killer cells superior CAR drivers? Oncoimmunology. 2014;3:e28147. doi: 10.4161/onci.28147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kriegsmann Katharina, Kriegsmann Mark, von Bergwelt-Baildon Michael, Cremer Martin, Witzens-Harig Mathias. NKT cells - New players in CAR cell immunotherapy? European Journal of Haematology. 2018;101(6):750–757. doi: 10.1111/ejh.13170. [DOI] [PubMed] [Google Scholar]
- 77.Chu J, et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia. 2014;28:917–927. doi: 10.1038/leu.2013.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jiang H, et al. Transfection of chimeric anti-CD138 gene enhances natural killer cell activation and killing of multiple myeloma cells. Mol. Oncol. 2014;8:297–310. doi: 10.1016/j.molonc.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leivas A, et al. Activated and expanded natural killer cells expressing an NKG2D-CAR efficiently target multiple myeloma cells. Blood. 2017;130(Suppl. 1):4466. [Google Scholar]
- 80.Ping Y, Liu C, Zhang Y. T cell receptor-engineered T cells for cancer treatment: current status and future directions. Protein Cell. 2018;9:254–266. doi: 10.1007/s13238-016-0367-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fesnak AD, June CH, Levine BL. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat. Rev. Cancer. 2016;16:566–581. doi: 10.1038/nrc.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dahan R, Reiter Y. T cell-receptor-like antibodies—generation, function and applications. Expert Rev. Mol. Med. 2012;14:e6. doi: 10.1017/erm.2012.2. [DOI] [PubMed] [Google Scholar]
- 83.Weidanz JA, Hawkins O, Verma B, Hildebrand WH. TCR-like biomolecules target peptide/MHC class I complexes on the surface of infected and cancerous cells. Int Rev. Immunol. 2011;30:328–340. doi: 10.3109/08830185.2011.604880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Debets R, Donnadieu E, Chouaib S, Coukos G. TCR-engineered T cells to treat tumors: seeing but not touching? Semin. Immunol. 2016;28:10–21. doi: 10.1016/j.smim.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 85.Maruta M, et al. Development of T cell therapy by exploiting modified antibodies specific for A2/NY-ESO-1 for refractory myeloma. Blood. 2017;130(Suppl. 1):1913. [Google Scholar]
- 86.Patel KK, et al. T cell therapy for multiple myeloma using NY-ESO-1+ T cell antigen presenting cells (T-APC) combined with adoptive cellular transfer (ACT) to augment immunotherapy. Blood. 2014;124:3843. doi: 10.1182/blood-2014-01-549352. [DOI] [Google Scholar]
- 87.Patel K, et al. Combination immunotherapy with NY-ESO-1-specific CAR+ T cells with T cell vaccine improves anti-myeloma effect. Blood. 2016;128:3366. [Google Scholar]
- 88.Mastaglio S, et al. NY-ESO-1 TCR single edited stem and central memory T cells to treat multiple myeloma without graft-versus-host disease. Blood. 2017;130:606–618. doi: 10.1182/blood-2016-08-732636. [DOI] [PubMed] [Google Scholar]
- 89.Rapoport AP, et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat. Med. 2015;21:914–921. doi: 10.1038/nm.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.van Rhee F, et al. NY-ESO-1 is highly expressed in poor-prognosis multiple myeloma and induces spontaneous humoral and cellular immune responses. Blood. 2005;105:3939–3944. doi: 10.1182/blood-2004-09-3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jahn L, et al. T cell receptors specific for the intracellular transcription factor Bob1 allow efficient targeting of human B cell leukemia and multiple myeloma. Blood. 2014;124:3832. [Google Scholar]
- 92.Jahn L, et al. T cell receptor gene therapy targeting the intracellular transcription factor Bob1 for the treatment of multiple myeloma and other B cell malignancies. Blood. 2015;126:3002. doi: 10.1182/blood-2016-09-737536. [DOI] [PubMed] [Google Scholar]
- 93.Jahn L, et al. TCR-based therapy for multiple myeloma and other B-cell malignancies targeting intracellular transcription factor BOB1. Blood. 2017;129:1284–1295. doi: 10.1182/blood-2016-09-737536. [DOI] [PubMed] [Google Scholar]
- 94.Hatta Y, et al. WT1 expression level and clinical factors in multiple myeloma. J. Exp. Clin. Cancer Res. 2005;24:595–599. [PubMed] [Google Scholar]
- 95.Rafiq S, Dao T, Liu C, Scheinberg DA, Brentjens RJ. Engineered T cell receptor-mimic antibody, (TCRm) chimeric antigen receptor (CAR) T cells against the intracellular protein Wilms tumor-1 (WT1) for treatment of hematologic and solid cancers. Blood. 2014;124:2155. [Google Scholar]
- 96.Yang L, Han Y, Suarez Saiz F, Minden MD. A tumor suppressor and oncogene: the WT1 story. Leukemia. 2007;21:868–876. doi: 10.1038/sj.leu.2404624. [DOI] [PubMed] [Google Scholar]