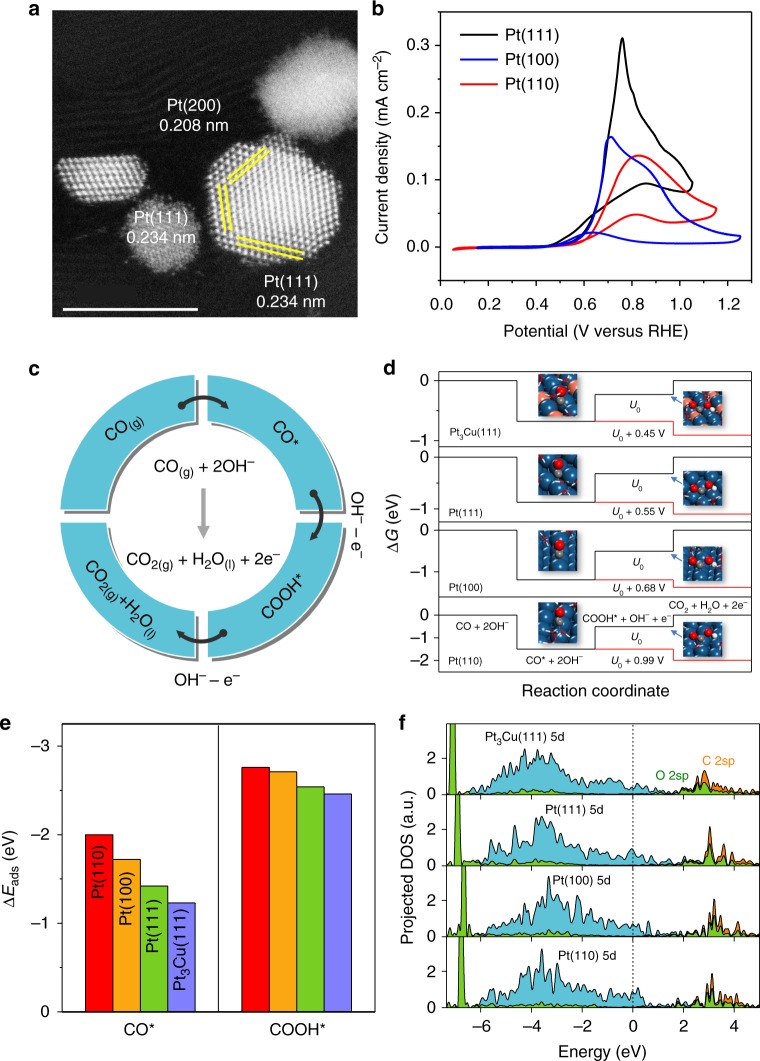
Fig. 3.
Insights into the reaction mechanism of different Pt facets towards the EWGS. a HAADF-STEM image of Pt@CNTs, scale bar: 5 nm. b CV test of Pt(111), Pt(110), and Pt(100) electrodes in CO-saturated 0.01 M KOH at 25 °C with a sweep rate of 50 mV s−1. c Reaction path of the EWGS process in alkaline solution. d Free energy diagrams of CO oxidation on Pt(110), Pt(100), Pt(111), and Pt3Cu(111) at the reversible potential (U0) of −0.16 V, and at the overpotentials that all reaction steps are downhill in free energy. e, f Comparison of the adsorption energies (ΔEads) of CO and COOH and projected density of states of CO on Pt(110), Pt(100), Pt(111), and Pt3Cu(111) in water environment