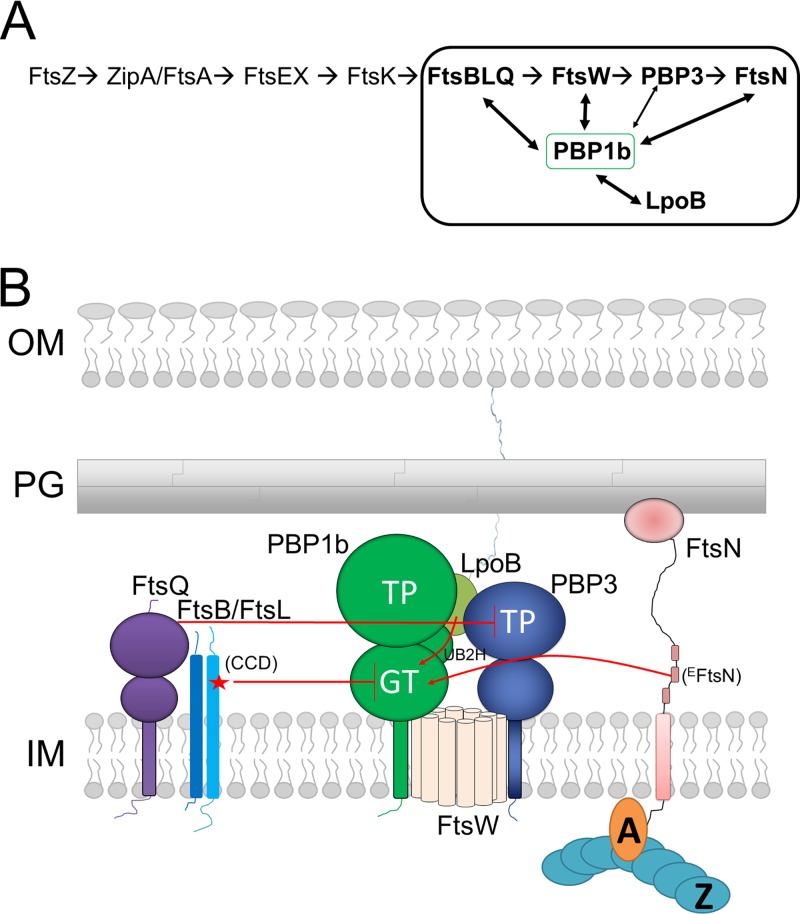
FIG 8.
Schematic representation of the linear recruitment pathway of the divisome and a regulation model of the septal synthase subcomplex (FtsW-PBP3-PBP1b) by FtsBLQ, FtsN, and the lipoprotein LpoB. (A) Linear recruitment pathway of the divisome with the septal peptidoglycan (sPG) synthesis core shown in a frame; protein-protein interactions are shown with arrows. (B) Topology of the synthase subcomplex and the regulatory proteins (FtsBLQ, FtsN, and LpoB) in the cell envelope. FtsBLQ subcomplex inhibits the GTase activity of PBP1b (via FtsL) and the TPase domain of PBP3 (via FtsQ). Interaction of FtsN via the essential region (EFtsN) and of LpoB with PBP1b suppresses the inhibition by FtsBLQ, activating PBP1b and probably the whole synthase subcomplex. Interaction of FtsN with FtsA and LpoB with the outer membrane allows the coordination of sPG synthesis with cytoplasmic, inner (IM), and outer membrane (OM) events. CCD, constriction control domain of FtsL.