Figure 5.
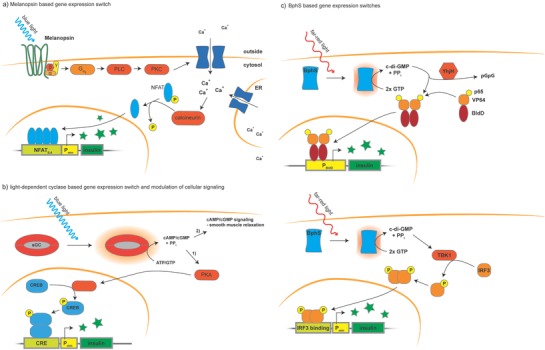
Optotherapeutic strategies: several optogenetic implants have already been tested in mice and proved to be functional to treat diabetes as well as erectile dysfunction. a) An implant harboring synthetic mammalian designer cells equipped with blue light–induced melanopsin signals through the protein kinase C (PKC) signaling pathway. Activation of melanopsin results in influx of Ca2+ ions from outside of the cells and release of calcium from the ER. Ultimately, this leads to activation of the phosphatase calcineurin, which dephosphorylates NFAT and triggers expression of insulin from a synthetic NFAT‐responsive promoter. b) To tackle the delicate issue of erectile dysfunction, a blue light–inducible soluble guanylate cyclase (sGC) of bacterial origin was engineered to favor GTP over ATP as a substrate. 1) To evaluate the performance of the system more easily in cell culture, cAMP levels were read out by the cAMP‐responsive CRE pathway. 2) Activation of the enzyme also directly leads to increased cGMP levels, triggering relaxation of smooth muscle cells. To avoid side effects, this system was not used as a cell‐based therapy, but was integrated directly into smooth muscle cells in the endothelium of the corpora cavernosum of rats. c) Two other approaches capitalized on the soluble c‐di‐GMP synthase BphS, which can be directly activated by far‐red light. In one system, c‐di‐GMP activated a chimeric transcription factor based on the bacterial protein BldD that dimerizes and binds to the DNA in a c‐di‐GMP‐dependent manner and is fused to the transactivators VP64 and p65. To avoid overproduction of c‐di‐GMP and overactivation of the system, YhjH was coexpressed; it hydrolyzes c‐di‐GMP to form inactive pGpG. In another study, c‐di‐GMP was used as an inducer of the endogenous STING pathway, which activates the transcription factor interferon regulatory factor 3 (IRF3) through phosphorylation by TANK‐inding kinase 1 (TBK1). Activated IRF3 binds to IRF3‐binding sites on a synthetic promoter and triggers insulin expression.
