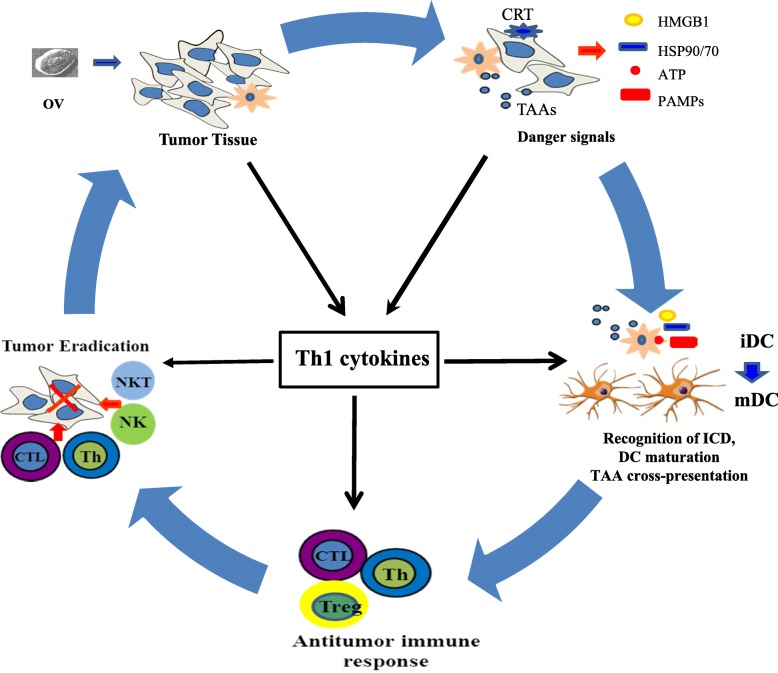
Fig. 2.
A model of how immunogenic cell death (ICD) and expression of proinflammatory Th1 cytokines from an oncolytic virus (OV) lead to potent antitumor immunity. An OV selectively replicates in tumor or/and stromal cells. This leads to induction of ICD, presenting both “find me” (extracellular HMGB1 and ATP) and “eat me” signals on the cell surface (such as ecto-CRT) to phagocytes. The presented/released danger signals (DAMPs and PAMPs) activate immature DC (iDC) to become mature DC (mDC). Apoptotic bodies and cellular fragments released via ICD are engulfed by APCs, and TAAs are processed into peptides that are presented in MHC class I/II complexes in concert with costimulatory molecules to naive CD8+ and CD4+ T cells, respectively. Such activated T cells may then expand and undergo polarized differentiation predictable on additional immune-stimulatory molecules expressed by recombinant OV. This figure has been modified from our previous model [6]. HMGB1: high mobility group box 1; DAMP: damage-associated molecular pattern; PAMP: pathogen-associated molecular pattern; APC: antigen-presenting cell; TAA: tumor-associated antigen