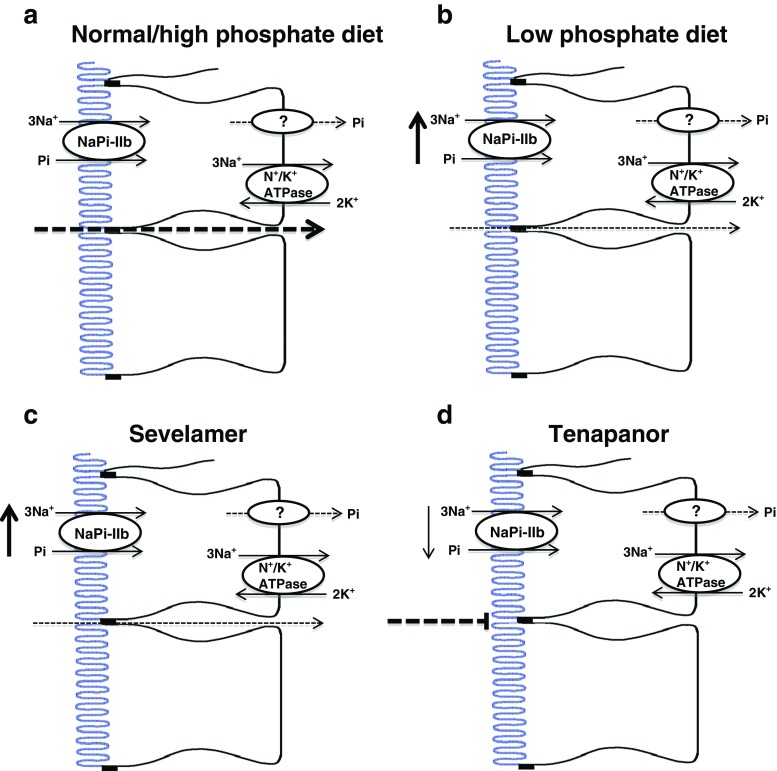
Fig. 1.
Potential adaptations in intestinal phosphate absorption in response to changes in dietary phosphate intake, or treatment with phosphate binders or the NHE3 inhibitor, tenapanor. a Ingestion of a normal or high-phosphate diet provides a luminal phosphate gradient that favours the paracellular pathway, with NaPi-IIb likely functioning at its maximal rate. Renal adaption occurs in response to the high dietary phosphate intake in order to maintain phosphate homeostasis [25, 51]. b In contrast, during ingestion of a low-phosphate diet, the luminal phosphate gradient is reduced and the contribution of the paracellular pathway diminishes. NaPi-IIb protein levels increase and this pathway becomes the dominant route for phosphate absorption [51]. Studies from NaPi-IIb−/− mice suggest that the adaptation in NaPi-IIb is required to protect bone during phosphate restriction [36]. c Phosphate binders reduce the luminal phosphate concentration and therefore the gradient for paracellular phosphate absorption. Recent studies in NaPi-IIb−/− mice have shown a potential compensatory upregulation in NaPi-IIb following sevelamer treatment [54]. d Tenapanor has been proposed to reduce paracellular phosphate absorption via changes in trans-epithelial electrical resistance. Interestingly, the inhibitor also induces a downregulation in NaPi-IIb protein levels as a means of preventing compensation by this pathway [34]. The basolateral pathway responsible for phosphate efflux from the enterocyte is unknown and whether it adapts during these conditions requires investigation