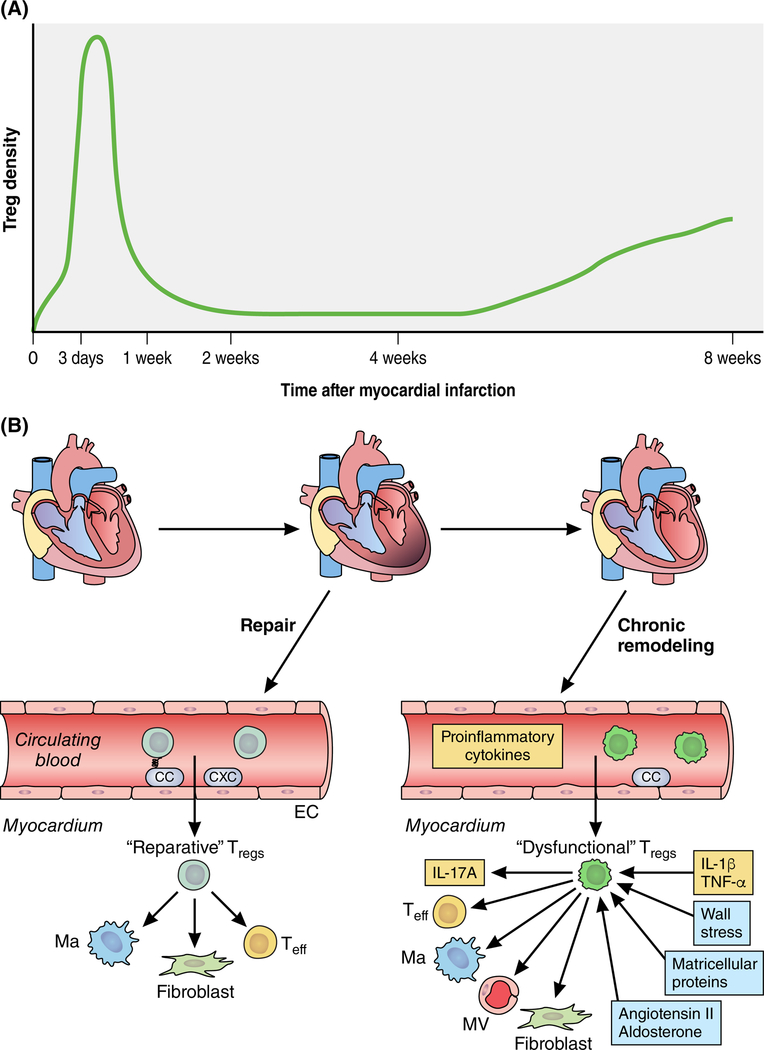
FIGURE 1: Dynamic alterations of regulatory T cell (Treg) phenotype may play an important role in repair and remodeling of the infarcted heart.
A. Tregs are recruited in the infarcted myocardium, exhibiting 2 “waves” of infiltration. The marked expansion of cardiac Tregs during the inflammatory and proliferative phase of infarct healing (3–7 days after coronary occlusion) is followed by a late, less striking increase in Treg density during the phase of chronic remodeling (8 weeks after infarction). B. Early recruitment of Tregs in the infarct is mediated through chemokine-dependent pathways3. “Reparative Tregs” play an important role in suppression of post-infarction inflammation and in repair of the infarcted heart by dowmodulating pro-inflammatory activity in macrophages (Ma) and effector T cells (Teff) and by activating fibroblasts (F)5,4. During the chronic phase of cardiac remodeling, Tregs have functional perturbations, exhibiting defective immunosuppressive and anti-inflammatory activity, producing pro-inflammatory cytokines, and exerting fibrogenic and angiostatic actions. The molecular signals promoting expansion of dysfunctional Tregs are poorly understood, but may involve chronic stimulation with pro-inflammatory cytokines, neurohumoral activation, and stimulation of Toll-like receptor signaling. The study by Bansal and co-workers6 demonstrates that dysfunctional Tregs play an important role in progression of adverse remodeling in chronic ischemic cardiomyopathy (see text). Additional symbols: EC, endothelial cell; MV, microvessel; CC, CC chemokine; CXC, CXC chemokine.