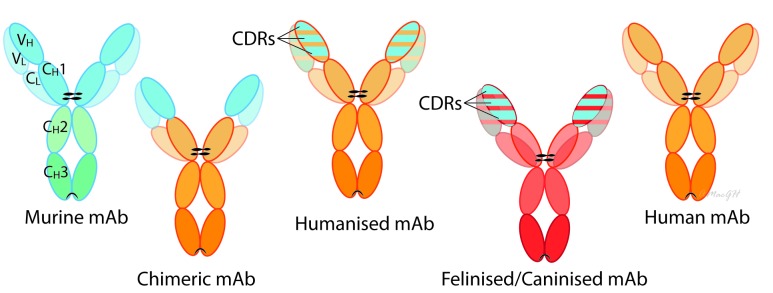
Figure 1.
Antibodies are large glycoproteins typically composed of two heavy chains and two light chains, each of which contain a variable domain (variable heavy (VH) or variable light (VL) and a constant domain (constant heavy (CH) or constant light (CL). The amino acid sequence of the variable domain varies greatly among antibodies and six ‘hyper-variable’ complementarity-determining region (CDR) loops within the variable domains give the antibody its specificity for binding to an antigen. In contrast, the constant domain is identical in all antibodies of the same isotype but differs in antibodies of different isotypes (IgA, IgD, IgE, IgG and IgM). The tail region of the constant domain (Fc region: CH2 and CH3) may direct immune effector functions by binding to cell receptors expressed on immune cells or initiating complementary-dependent cytotoxicity. Murine monoclonal antibodies (mAbs) are antibodies produced from individual cloned and immortalised mouse B cells. Most useful therapeutic antibodies have been constructed with the gamma immunoglobulin (IgG) isotype. Chimeric mAbs are antibodies made by fusing the genes encoding the variable region from a murine-derived mAb, with those from an immunoglobulin (Ig) constant region from a human antibody. Humanised mAbs retain only the CDRs (part of the variable domain from the original murine-derived mAb that binds to the specific antigen). Fully human mAbs have no murine sequences. Caninised and felinised mAbs are fully canine or feline specific. These can be made in several ways; for example, Nexvet have used a process of conversion based on alignment with immunoglobulin complementary DNA libraries (PETization).