Abstract
Management of chronic conditions is a challenge for healthcare delivery systems world over and especially for low/middle-income countries (LMIC). Redesigning primary care to deliver quality care for chronic conditions is a need of the hour. However, much of the literature is from the experience of high-income countries. We conducted a synthesis of qualitative findings regarding care for chronic conditions at primary care facilities in LMICs. The themes identified were used to adapt the existing chronic care model (CCM) for application in an LMIC using the ‘best fit’ framework synthesis methodology. Primary qualitative research studies were systematically searched and coded using themes of the CCM. The results that could not be coded were thematically analysed to generate themes to enrich the model. Search strategy keywords were: primary health care, diabetes mellitus type 2, hypertension, chronic disease, developing countries, low, middle-income countries and LMIC country names as classified by the World Bank. The search yielded 404 articles, 338 were excluded after reviewing abstracts. Further, 42 articles were excluded based on criteria. Twenty-four studies were included for analysis. All themes of the CCM, identified a priori, were represented in primary studies. Four additional themes for the model were identified: a focus on the quality of communication between health professionals and patients, availability of essential medicines, diagnostics and trained personnel at decentralised levels of healthcare, and mechanisms for coordination between healthcare providers. We recommend including these in the CCM to make it relevant for application in an LMIC.
Keywords: chronic care, model, LMICs, diabetes, hypertension, evidence synthesis, primary care
Key questions.
What is already known?
Healthcare systems in low/middle-income countries (LMIC), traditionally, are geared towards care for acute conditions and now need to respond to the increased burden and challenge of chronic care.
What are the new findings?
The elements of the chronic care model, namely the community, the health system, self-management support, delivery system design, decision support and clinical information systems, are all relevant to care in LMICs, even though they have been applied differently to fit the context.
The model additionally requires a focus on: the quality of communication between health professionals and patients, emphasis on the availability of essential medicines, diagnostics and trained personnel at decentralised levels of healthcare, and mechanisms for coordination between healthcare providers.
What do the new findings imply?
An adapted chronic care model with the additional themes identified could guide organisation of chronic care in LMIC settings. Implementation research is required to test the effectiveness of interventions that operationalise the suggested model of care in LMICs.
Introduction
Globally, chronic non-communicable diseases (NCD), mainly cardiovascular diseases, diabetes, chronic lung disease and cancers, have emerged as the leading causes of death. They together accounted for 72.3% of deaths in 20161 and according to the WHO, 86% of premature deaths occurred in low/middle-income countries (LMIC) like India.2
The growing burden of chronic disease is a huge challenge for healthcare services world over.3 Persons with chronic diseases require sustained engagement with the healthcare delivery system over the course of their lives. They also need support for skills to manage their disease condition more than would be required for an acute health condition. Primary healthcare has been envisaged as the level of care closest to the patient from where this can be facilitated.4 However, primary care, especially in LMICs, is traditionally geared to respond to acute healthcare needs and therefore, a redesign of primary care that responds to the challenges of chronicity is a need of the hour.5
The chronic care model (CCM) developed in the 1990s by Wagner et al 6 is a dominant framework in literature, which is considered to be effective in guiding the design of services for chronic conditions.7 It envisions meaningful, productive interactions between patients and healthcare teams resulting in good outcomes. However, as Beaglehole et al point out, most studies on the organisation of services to deliver quality chronic care are from high-income countries and frameworks such as the CCM may not be directly applicable to LMICs.8 The unique challenges these countries face such as lack of medically qualified staff and laboratory support for basic biochemistry among other constraints8 are usually not considered in these models of care. Therefore, the application of such models of care is limited if not adapted to the specific context and constraints of LMICs.9
In this study, we aimed to analyse the CCM for its relevance to LMICs and also sought to adapt it for application in LMICs. The healthcare delivery systems in LMICs, though different from each other, have some commonalities that enable learnings to be shared and applied across these countries.10
The ‘best fit’ framework synthesis (BFFS) is a relatively new method of synthesis that offers a means to test, reinforce and build on an existing model, such as the CCM, which was developed for a potentially different but relevant population.11 Synthesising results from multiple similar studies allows us to develop generalisable conclusions that may not be possible from a single study.12 We use this novel method to synthesise the results from primary qualitative research in LMICs regarding experiences of patients and providers in seeking and delivering chronic care. This method is a pragmatic approach to answer policy and managerial questions such as how do we deliver good quality care for diabetes and hypertension at the primary level of care in India.13 14
We chose to focus on diabetes and hypertension in this review as the delivery of services for these share commonalities in opposition to cancers and lung diseases, which are also chronic diseases, but with greater complexities. However, in theory, the adapted model should be flexible enough to respond to most chronic diseases.
Methods
We used the BFFS methodology as it was well suited to the aim of the review. The purpose of the synthesis was to build on an existing model for chronic care at the primary care level, rendering it relevant to an LMIC.
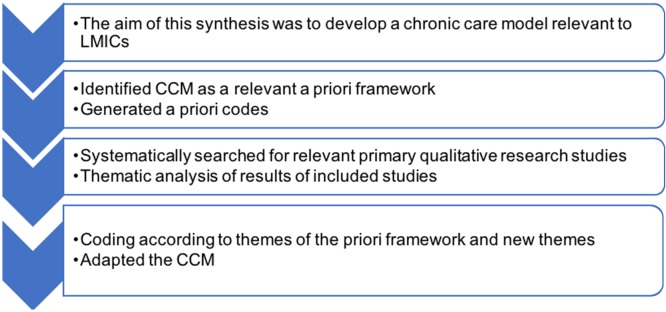
Framework synthesis is based on the principles of framework analysis of primary qualitative research data.15 Framework analysis is a well-established method of qualitative data analysis, which involves the use of codes developed from a framework or a model identified prior to analysis.15 The qualitative research data are thematically analysed and coded into prespecified themes. Framework synthesis, therefore, is distinct from other methods of synthesising qualitative research, in that it uses an a priori framework to identify and extract themes. The BFFS constitutes an innovation of framework synthesis in that it also includes thematic analysis of results from studies that cannot be coded or do not ‘fit’ the themes identified a priori.16 The new themes that emerge enable the development of a new framework or enrich the existing a priori framework. Carroll et al describe the methodology in detail (figure 1)11 14 and the following steps are involved: (1) identifying a suitable conceptual framework relevant to the aim of the synthesis; (2) deriving themes from the framework that can be used for coding; (3) systematically searching relevant databases to identify relevant primary research studies for inclusion; (4) coding the results of the studies included; and (5) thematic analysis of the results of studies included that could not be coded to identify new themes. The final product is a new or revised framework that includes both the relevant a priori themes and the new themes that were not captured in the original framework.16
Figure 1.
Steps in conducting the best fit framework synthesis. CCM, chronic care model; LMIC, low/middle-income country.
The a priori framework
We searched the literature for CCMs and found several systematic reviews of CCMs.9 17 18 The most dominant evidence-based model reported was the CCM developed at the McColl Institute by Wagner et al 6 among other models such as the integrated chronic care framework of the WHO, which is also an adaptation of the CCM.19 We selected the CCM as the a priori framework for this review as it is one of the most widely used models of provision of chronic care in primary care settings.7 20 Many national health authorities have found inspiration in this model to develop their chronic care programmes.21 We, therefore, did not repeat a systematic search of CCMs but chose to derive the a priori codes for the framework synthesis from the CCM.
The CCM is an organisational approach to caring for people with a chronic condition in the primary care setting. It identifies key actors and essential elements of a healthcare system that encourage high-quality chronic disease care: the community, the health system, self-management support, delivery system design, decision support and clinical information systems.6 These elements are described by the authors of the CCM as specific change concepts that should guide processes in redesigning primary care.22 We studied the description of these change concepts as defined by the authors to identify codes for analysis of the primary research studies (table 1).
Table 1.
A priori codes identified from the CCM
| CCM elements | Change concepts | Codes |
| Health systems Create a culture, organisation and mechanisms that promote safe, high-quality healthcare |
Leadership motivated to make improvements and handle errors systematically | Organisational culture for safety and quality |
| Self-management support Empower and prepare patients to manage their health and healthcare |
Self-management support strategies to enable goal setting, action planning and regular follow-up emphasising patients’ central role | Support for self-management |
| Delivery system design Assure effective, efficient clinical care and self-management support |
Clearly define roles and distribution of tasks among the healthcare team for planned interactions | Planned interactions based on defined roles of team |
| Decision support Promote clinical care consistent with scientific evidence and patients’ preferences |
Enable use of evidence-based guidelines through provider education | Guidelines and provider education |
| Clinical information systems for individual care plan Organise patient and population data to facilitate efficient and effective care |
Provide timely reminders for follow-up and identify high-risk patients for appropriate action | Follow-up care |
| Community resources and policies Mobilise community resources to support patients |
Form partnerships with community organisations to support and fill gaps in health service provision | Community linkages |
CCM, chronic care model.
Search strategy
The next step in this review was to identify primary qualitative research studies that described experiences in seeking and providing care for chronic conditions in LMICs. We included qualitative research studies that were from an LMIC, focused on healthcare for diabetes and hypertension at primary care level, and with results that described experiences, barriers and enablers of care at these health facilities. We excluded studies that were not related to primary healthcare and those that primarily described illness experiences or coping with these conditions not in the context of service delivery (table 2). We searched the three most relevant electronic databases of health literature—PubMed, Embase and CINAHL (Cumulative Index to Nursing and Allied Health Literature). We also identified articles from reference lists.
Table 2.
Characteristics of studies included in the review
| Study number | Study | Country | Method for data collection | Participants and sample size for interviews/FGDs | Research question and analysis | Setting/context of study—healthcare services and utilisation |
| Asian region | ||||||
| 1 | 31 | South India | In-depth interviews | Patients with DM n=16 | Constraints faced by patients in managing care for diabetes, thematic analysis | Government health centres’ free care and private fee-for-service facilities. Urban area. |
| 2 | 29 | South India | Observations, semistructured interviews |
Specialist and non-specialist doctors, pharmacists and laboratory technician n=19 | Organisation of a local health system for chronic care, thematic analysis |
Mixed health system-health centres, clinics and hospitals in an urban slum area |
| 3 | 40 | India | Semistructured interviews | Patients with a diagnosis of DM, HTN, TB n=7, FGDs with TB n=12, diabetes n=18 and HTN n=27 | Patient experiences in diagnostic services, thematic analysis |
Pluralistic healthcare services—public PHCs providing free OP care and many private providers |
| 4 | 32 | Bangladesh | In-depth interviews | Patients with a diagnosis of DM n=23 | Patient experiences of care for DM, thematic analysis | Diabetes Association of Bangladesh (BADAS) provides specialist clinics and tertiary-level specialist hospitals. |
| 5 | 59 | Vietnam | In-depth interview and FGDs | Health staff, patients with NCDs and relevant stakeholders at 20 centres | Commune health stations capacity for NCDs, content analysis |
A national strategy to have 90% of health facilities at the primary healthcare level with essential medical products and technology |
| 6 | 26 | Mongolia | In-depth interviews, FGD |
Practice doctors and practice directors at PHCs treating HTN n=10 | Factors influencing primary care providers’ role delineation in guideline implementation, thematic analysis | State-funded Family Health Centres provide universal access to healthcare for individuals, families and communities. |
| 7 | 44 | Mongolia | Semistructured interviews, FGDs | Nurses n=20, practice doctor n=10 and practice managers n=10 |
Implementation of guidelines at primary care, thematic analysis using theoretical domains framework |
Family health centres’ private entities funded by the government. Services free of charge for citizens. Ministry publishes clinical guidelines for HTN and DM. |
| 8 | 45 | Cambodia | In-depth interviews | Patients with a diagnosis of DM and/or HTN n=28 | Patient experiences in care for DM and HTN, grounded theory | Public chronic disease clinics at provincial and district hospitals. Also, private providers. |
| 9 | 33 | Malaysia | In-depth interviews | Patients with HTN n=25 | Patient experiences of chronic care and self-management, thematic analysis |
Chronic disease primary health centres run by the government |
| 10 | 41 | Malaysia | Document review and semistructured interviews | Patients with a diagnosis of HTN n=37 and health providers n=24 | Barriers and facilitators for hypertension management, thematic analysis |
Ministry of Health guidelines, staff training in screening and HTN management, traditional complementary medicine widespread |
| African region | ||||||
| 11 | 28 | Tunisia | Participant observation, semistructured interviews, FGD | Patients n=12 Paramedical staff n=4 Clinicians public sector n=10 Observations n=50 centres |
Barriers and facilitators of care in the management of DM, content analysis |
Ministry of Health—programme for management of HTN and DM in primary care. Public and private health sectors coexist. |
| 12 | 34 | Tunisia | Semistructured interviews | Patients with DM or HTN n=24 | Patient experiences of chronic care, thematic analysis | Government-run primary health centres |
| 13 | 60 | South Africa | In-depth narrative interviews and survey | Women with self -reported DM/HTN n=12 | Facilitators and inhibitors of healthcare utilisation for DM and HTN, thematic analysis | Healthcare system historically inequitable due to a racially fragmented public healthcare approach. Underutilisation of services. |
| 14 | 35 | South Africa | In-depth interviews | Patients with DM/HTN n=22 | Patient experiences of chronic care and self-management, framework analysis using self-determination theory |
National Department of Health patient-centred model of chronic care and free primary healthcare |
| 15 | 42 | South Africa | In-depth interviews | Patients with DM n=31 and healthcare providers n=23 | Reasons for missed appointments at PHC, thematic analysis | Chronic Dispensing Unit at PHC>75% dependent on the public sector for medicines |
| 16 | 30 | South Africa | FGDs | Patients with DM and providers n=10–12 | Barriers and facilitators of chronic care, thematic analysis | Primary care community health workers and traditional healers provide services. |
| 17 | 36 | Kenya | FGDs and in-depth interviews | Patients with DM or HTN n=179 and 4 FGDs n=242 | Factors influencing linkage to HTN care, thematic analysis | Clinics of AMPATH and Kenya government, optimising referral and retention in care |
| 18 | 46 | South Africa | In-depth interviews | Women with DM n=27 | Patient experiences with chronic care, thematic analysis | Public and private healthcare delivery with low utilisation of healthcare due to systemic inequalities |
| 19 | 37 | Zambia | In-depth interviews | Healthcare providers n=20 in 46 clinics | Assess care delivery at centres enrolled in an intervention study, thematic analysis |
Better Health Outcomes through Mentoring and Assessment, 5-year trial of improved clinical service delivery in rural government clinics. |
| 20 | 27 | Nigeria | Semistructured interviews | Physicians, nurses, pharmacy staff, laboratory staff, administrative staff of health centres treating HTN n=39 | Factors that inhibit or facilitate high-quality care, framework analysis using the tailored implementation for chronic disease framework |
State Health Insurance clinics—a voluntary community-based health insurance programme supports quality improvement, provides new equipment, organisational support and staff training. |
| South American region | ||||||
| 21 | 25 | Mexico | In-depth interviews | PHC personnel including physicians, nurses and directors n=105 | Patient experiences in HTN management and control, framework analysis |
Casalud—comprehensive NCD care model based on the use of patient-centred technologies implemented through a public–private partnership |
| 22 | 39 | San José, Costa Rica, Mexico | FGDs | Patients with DM and/or HTN at urban public health centres n=70 in 12 FGDs | Patient perception of barriers and facilitators to self-management | Secretary of Health programme of healthcare for all, Costa Rica—a comprehensive healthcare system |
| 23 | 38 | Colombia | In-depth interviews, FGDs |
Patients with HTN n=26, patients n=6 and family members n=4 |
Patient experiences in management and control of HTN, thematic analysis |
The mandatory mixed contributory scheme covers salaries of retired and subsidised health insurance regime for the poor. |
| 24 | 43 | Brazil | In-depth interviews | Physicians, nurses, ANMs, community health agents and other staff at PHC n=38 | Care provided by health professionals from a perspective of country policy, framework analysis |
Brazilian Health Department uses the chronic care model as the main reference for the construction of the Modelo de Atenção às Condições Crônicas Healthcare Networks. |
AMPATH, Academic Model Providing Access to Healthcare Partnership; ANM, Auxillary Nirse Midwife; DM, diabetes mellitus type 2; FGD, focus group discussion; HTN, hypertension; NCDs, non-communicable diseases; OP, Out Patient; PHC, primary health centre; TB, Tuberculosis.
The search terms for PubMed were: primary health care [MeSH], delivery of healthcare [MeSH], primary health, preventive health services [MeSH] AND diabetes mellitus type 2, hypertension and chronic disease [MeSH]. We selected studies documented in English and used the date range 1 January 2007 to 31 December 2017 as a filter for the search. We chose to select studies published over the last 10 years as we felt these would fit reasonably well the current context. The World Bank classification of LMICs for 201723 was used to identify names of all countries in this category and these were used with the above terms in the search strategy. We also used additional terms such as developing countries and low-income countries to widen the search. The strategy was created for PubMed and modified to suit the requirements of the Embase and CINAHL databases.
Analysis
The selected studies were uploaded to NVivo database (NVivo qualitative data analysis software; QSR International, V.10; 2012). The results reported in these selected studies were coded according to the a priori coding scheme (table 1) using a deductive approach. Data for analysis consisted either of verbatim quotations from study participants or findings reported by authors that were clearly supported by study data. The results that could not be coded were thematically analysed and coded inductively. The codes were discussed among the authors and finalised in an iterative manner. The main themes not captured in the a priori framework were related to: (1) poor communication between healthcare providers and patients; (2) distance and cost of care impacting continuity of care; (3) availability of essential medicines, essential diagnostics and trained staff at healthcare facilities; and (4) lack of coordination of care across public, private and alternative providers of healthcare.
Quality appraisal
We conducted an appraisal of all the included studies for the robustness of methods using the Critical Appraisal Skills Program checklist24 for qualitative research. All the studies were found to be of reasonable quality and findings were supported by the data that were appropriately collected and analysed.
Results
The search yielded 404 articles (figure 2; Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart) after removing duplicates. We excluded 338 articles that were not relevant from reading the title and abstract. Full texts of 66 articles were reviewed and 42 of these were excluded as they were either not related to primary care (n=22), not from an LMIC (n=6) or were protocol papers (n=4). Eventually, 24 studies were included for thematic analysis and coding. The majority (n=10) of the included studies were from Asia (India, Cambodia, Vietnam, Mongolia and Malaysia) and from the African region (n=10) (Tunisia, South Africa, Kenya, Nigeria and Zambia). The remaining (n=4) were from South America (Colombia, Brazil and two from Mexico). Most of the studies used in-depth interviews to collect data that were thematically analysed (table 2.)
Figure 2.
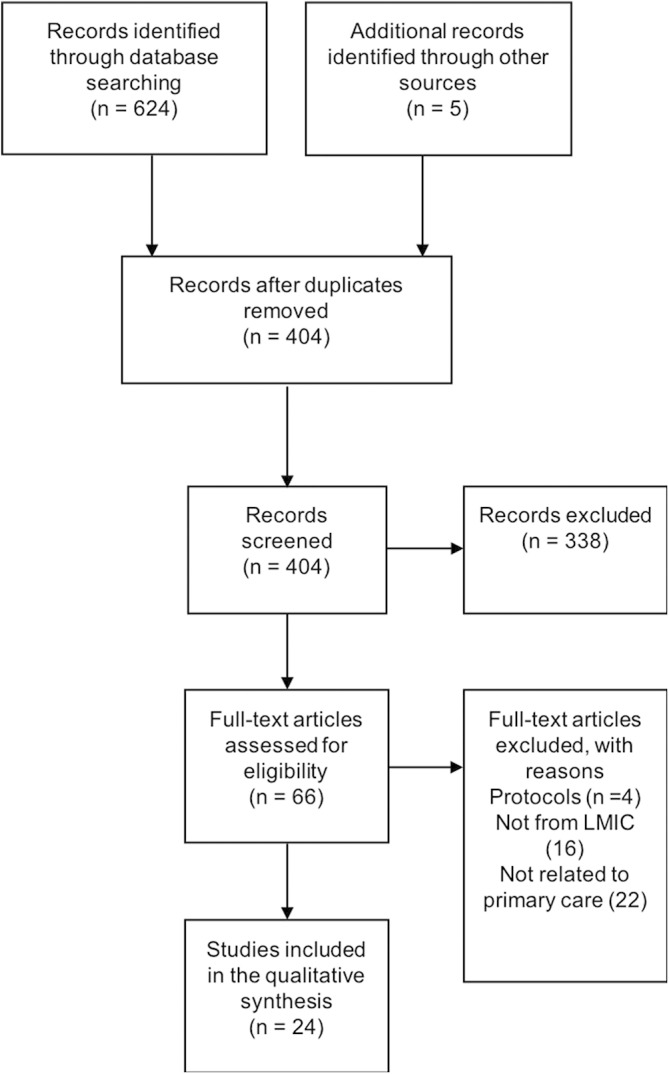
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart. LMIC, low/middle-income country.
The studies were coded along the lines of the a priori framework and we found that all the a priori codes were represented in at least one study (table 3).
Table 3.
A priori themes reflected in the primary research studies included in the review
| Study reference | Organisational culture for safety and quality | Support for self-management | Planned interactions based on defined roles of team | Guidelines and provider education | Follow-up care | Community linkages |
| 31 | ||||||
| 29 | ||||||
| 40 | ||||||
| 32 | ||||||
| 59 | ||||||
| 26 | ||||||
| 44 | ||||||
| 45 | ||||||
| 33 | ||||||
| 41 | ||||||
| 28 | ||||||
| 34 | ||||||
| 60 | ||||||
| 35 | ||||||
| 42 | ||||||
| 30 | ||||||
| 36 | ||||||
| 46 | ||||||
| 37 | ||||||
| 27 | ||||||
| 25 | ||||||
| 39 | ||||||
| 38 | ||||||
| 43 |
Shaded area reflect the themes of the CCM captured in the studies included for the review.
Organisational culture for safety and quality was included in six studies. The CCM describes this element as visible support for care improvement at all levels of the system including senior leaders. Senior leadership translates care improvement into clear goals and policies. A study from Mexico, which implemented the CCM, found the support of key leaders to be an important enabler of the prevention and care of NCDs.25 Creation of quality assurance programmes and quality monitoring teams demonstrated a high organisational commitment to improvement as described in the CCM.25–27 Similarly, the introduction of weekly chronic disease clinics was perceived by doctors and patients to improve quality.28 Additionally, in some studies, the lack of a functional platform for grievance redressal and shortages of the health professionals’ time were considered barriers to quality improvement.29 30
Support for self-management appeared as a dominant theme that was part of the narrative in 14 of the 24 studies. According to the CCM, this is described as emphasising the central role of the patient and empowering her/him with skills to manage disease and sustain lifestyle changes. Most studies reported that, from a patient’s perspective, doctors and healthcare professionals do not have time to explain medication and lifestyle modification in busy outpatient clinic settings.31–38 Patients also feel that not enough information is tailored to their specific social and economic situation.32 39 From the providers’ perspective, patients are unable to follow and adhere to the advice they give owing to low levels of health literacy, inappropriate education materials and lack of motivation to change behaviour.26 29 30 34 35 40–43 Providers also acknowledge the lack of time and of trained staff as barriers to providing self- management support.37 Patients are unsure of what exactly is required of them and are not involved in decision-making. This is also because traditional models of provider–patient interaction prevail with the provider assuming a dominant role and determining the agenda of the consultation.34 35
These studies sketch how patients, even though, from different contexts, expressed a lack of confidence in undertaking self-care; they look to healthcare providers for assistance and were disappointed when this was not forthcoming.
Planned interactions with the team were captured in seven studies and this concept is described in the CCM as determining what sort of care is needed and identifying roles and tasks to ensure that patient interactions are structured and planned. The nurse and the pharmacist, other than the doctor, had active roles in patient care at most health centres. However, these were not always clearly delineated and planned but taken up on an ad hoc basis, dependent on workload and the explicit demands of patients.26 28 30 37 41 The nurse was perceived by patients as the best person to give counselling and support self- management,28 30 35 and in some settings, such as Africa, the nurse was the primary care provider. In all the three regions (Asia, Africa and South America), the doctor played a pivotal role in patient care both from patients’ and other health professionals’ perspectives. The contributions of other health workers and disciplines at the health facility were perceived as supportive to the doctor’s central role. Doctors, however, valued the teamwork approach and specifically looked at nurses to take up an active role in patient care.26 44
Guidelines and provider education to enable sound treatment decisions were included in seven studies. In most of the studies, guidelines were not used consistently by providers for various reasons including lack of awareness, guidelines not being practical for the local context and lack of time to put them into practice.26 27 29 37 43 44
Follow-up care facilitated by clinical information systems was reported in six studies. These studies reported a range of experiences with health information recording and follow-up care. The facilities report using patient-retained health information booklets, facility-based manually recorded folders for each patient and recording books for patient visits and appointments.27 29 41 However, poor clinic attendance was a challenge in most of the studies, as patients attend when they feel the need to seek care and not because an appointment was given.32 37 38
Community linkages are described in the CCM as efforts to mobilise community resources to meet people's needs. Most studies report a lack of such resources in the community. Even though the context of each study was varied, the family was most often reported as the only social support available.38–40 42 45 However, patients express the desire for home-based caregivers to provide medication at home,46 give information and education30 and provide a link to social welfare schemes.30 41 In the African region, informal (mostly home-based) carers assisted professionals by tracing patients lost to follow-up or by referring new patients to the health facility. In the studies pertaining to the Asian and South American regions, this role of informal carers or community health workers was not reported.
The results of primary research studies that could not be coded were thematically analysed and additional themes were identified.
Poor communication between healthcare providers and patients
The relationship and communication with the healthcare provider emerged as an important consideration for patients. It had an effect on compliance to medication and continuity of care. The design of service delivery in most facilities was such that it did not enable meaningful interactions with the healthcare team. Nine of the 24 studies reported that patients were not satisfied with the time and the quality of communication with healthcare providers.27 31 33 35 36 38 39 43
A quote from a study conducted in India investigating the constraints faced by the urban poor in managing diabetes is illustrative of this poor communication.
He [a doctor] does not explain anything. As soon as I go there, he will write a prescription, take his fees and send us away …. We are uneducated, so we will simply sit quietly.31
Some studies report harsh words and impolite manners of doctors and nurses29 31 38 attributed to the high workload at primary care facilities and the poor communication skills of doctors.26 28 33 35 44
Distance to a health facility and inability to afford costs impacting continuity of care
Even when services for the care of persons with diabetes and hypertension were provided at primary care facilities, the distance to the facility impacted patient's ability to maintain regular follow-up. This theme emerged from seven studies where patients narrate that maintaining regular visits is difficult due to the large distances, more in rural areas where there were no medical services to meet even their basic needs.27 32 34 36 38 40 41
One of these studies, conducted in India, investigated the work patients need to do in order to seek care for diabetes also report:
The long distance, transportation costs involved and loss of wages prevented 25 patients from regularly going for follow-up visits to monitor their blood sugar levels.40
In addition to distance, cost of accessing care or paying for drugs, the doctors’ fees also deterred patients from remaining in regular care. The patient’s inability to purchase care for their chronic condition was a strong barrier to regular follow-up and care that was reported in 13 studies.26–29 31 32 34 36 38 40 42 45
In a few studies, where health insurance was provided to persons with diabetes, there was better compliance with drugs and regular visits as supported by this finding from a study in Nigeria that evaluated the management of hypertension at rural primary care facilities.
The insured patients are better compliant with visits, drugs and advice and are better controlled.
This has a lot to do with the fact that barriers to access care have been removed by the insurance.27
Availability of essential medicines, essential diagnostics and trained staff at healthcare facilities
Resource availability in terms of medicines, equipment, laboratory supplies and personnel is needed for provision of health services. In many of the studies included, challenges with respect to the availability of these basic resources emerged as barriers to the delivery of care.28 30–32 36 37 45 46 Shortages in medicines were common especially at publicly funded facilities like in the following example from a study in Tunisia that explored perspectives of healthcare providers on chronic disease management.
We always ask the patient to take his drugs, and we are always saying to him never to stop. But then we say to him, ‘no, we don’t have [these] drugs, come back tomorrow.’34
The lack of functional basic equipment such as those required for blood pressure measurement or blood sugar estimation was reported.37 The finding from the facility assessments in a mixed methods study from Malaysia is illustrative of this:
The facility checklists showed …that rural clinics had inadequate equipment of all types, both in terms of supply and quality, with much being very old.41
Lack of coordination of care across public, private and alternative providers of healthcare
The health systems in most LMICs are pluralistic, having both public and private sectors, and mixed with different systems of medicine (modern and traditional) providing services for diabetes and hypertension. The choice of where to seek healthcare is largely driven by the patient's ability to pay for services. Many patients seek care from multiple service providers and a lack of coordination between these different healthcare providers impacts continuity of care and regular follow-up.28–31 34 36 38 40 42 45 46 Studies from the African region have reported herbalists and traditional healers as important healthcare providers, often referring patients when they were seriously ill.30 In the Asian region, naturopathy, Ayurveda, yoga and homeopathy were commonly providing care for chronic diseases.
A study done in South Africa investigated reasons for missed appointments and found that patients seek care from multiple providers and this impedes regular visits to one healthcare provider.
Self-treatment with alternative medication sourced from informal providers (herbalists and traditional healers) was reported and use of plural healthcare sources could result in missed appointments.42
Discussion
We conducted a BFFS with the aim of critically analysing the appropriateness of the CCM for an LMIC. We found that while all the elements of the CCM are relevant to LMICs, there were a number of additional elements that need emphasis: the need to focus on the quality of communication between health professionals and patients, service provision at decentralised levels of healthcare, the availability of essential medicines, diagnostics and trained personnel, and coordination between the many healthcare providers.
The studies in this review were from the Asian, African and South American regions. In most of the countries, despite challenges and constraints,47 48 there have been initiatives from the ministries of health to provide services for NCDs. The initiatives to improve care, however, lack a systemic approach except for the Casalud—a comprehensive NCD care model in Mexico,25 and the Modelo de Atenção às Condições Crônicas in Brazil.43 A model of care broadly defines the way health services are delivered and enables systematically planned delivery. It also reinforces that multiple areas need to be addressed for better outcomes rather than single interventions such as only providing guidelines for better case management.49
Most models of care for chronic conditions have not taken into account the unique challenges of LMICs.8 50 A recent review of CCMs relevant for sub-Saharan Africa51 reported that themes such as staff competence, dedicated NCD staff, review criteria and communication with medical doctor/specialist were not captured in most care models.51 Similarly, a review that studied the experiences of innovative models of care and initiatives for diabetes in LMICs identified collaboration, education, standardisation of guidelines, resource optimisation and technological innovation as principles for better outcomes.52
The CCM includes developing an organisational culture of quality and safety, support for self-management, planned interactions with the team, guidelines and provider education, follow-up care and community linkages.6 Based on the findings of our qualitative synthesis, we recommend that the CCM include an emphasis, first, on the quality of communication between health professionals and patients. This is not explicitly stated in the CCM but is essential to providing support for self-management and also underlies the element of delivery design where interactions with the team are planned.53 In all the three regions, the doctor had a pivotal role in the interactions, however, the enhanced role of the nurse in the African regions can potentially provide important lessons for other LMICs. This emphasis on communication is especially relevant in LMICs as many factors unique to the context such as the volume of patients and poorly resourced health facilities contribute to poor communication.54 A focus on patients and building relationships for long-term engagement are central to the quality of care and to the concept of primary healthcare, in agreement with the proposition that health systems are essentially relational.55
Second, service provision at decentralised levels of healthcare has been associated with improved health outcomes56 and needs to be continually emphasised. Therefore, we recommend including this explicitly in the CCM for LMICs and suggest positioning it within the broader health system element of the current CCM. The reality in most LMICs is that the vision of accessible services, close to people's homes, from where care can be coordinated4 is still a distant dream.57
Third, the availability of essential medicines, diagnostics and trained personnel is a known challenge for most LMICs. Including this as an element of the care model could ensure attention to these basic requirements for the provision of care.58 Most health systems in LMICs are not designed for the care of chronic conditions and so lack a comprehensive approach to ensuring their availability.
And lastly, coordination between the many healthcare providers is very relevant for LMICs and should be explicitly stated in the model. There is a multiplicity of care providers and patients access several providers for the same chronic condition,29 therefore, enhancing coordination between them is vital for good quality continuous service provision. This would require creating platforms for increased engagement between different providers, across different systems of medicine, especially in the Asian region. In the African region, the traditional healthcare provider and the herbalist need to be acknowledged and involved to ensure timely referrals. Developing well-defined referral processes to coordinate care across different levels of care between specialists and generalist providers, as well as between public and private healthcare providers, is much needed.
Innovative ways in which these elements can be implemented at primary care in different local contexts need to be tested. Kruk et al identify team care with task shifting and harnessing information and communication technology as potentially generalisable opportunities to implement any care model.5 This review identifies these additional elements as potential areas for implementation research to understand how these can be operationalised in the real-world setting.
The BFSS methodology allowed us to use qualitative research findings to adapt the existing CCM. The experiences of patients and providers were used to enrich the existing model and increase its relevance to an LMIC. Another strength of the methodology used is the systematic search of the literature, across three databases, to identify the 24 primary research studies. However, we cannot exclude that we may have missed relevant studies since we limited ourselves to studies published in the last 10 years. Another limitation of our work is that we did not conduct a review of the various existing CCMs but opted for the start on for the CCM to derive the a priori themes. It is possible that there may be models that incorporate some or all of the additional themes we have proposed. Lastly, a limitation inherent to the methodology we used is that the qualitative studies we investigated are context specific and their results therefore difficult to be generalised. The thematic analysis nevertheless allowed us to identify themes and draw lessons that appeared to cross-cut different contexts.
Conclusion
There is a need to redesign primary care services to respond to the growing burden of chronic NCDs in LMICs. This qualitative synthesis of patient and provider perspectives of chronic care, in LMICs, highlights additional considerations for the CCM. We recommend including communication between health professionals and patients, service provision at decentralised levels of healthcare, the availability of essential medicines, diagnostics and trained personnel, and coordination between the many healthcare providers as other essential elements in the CCM. These could guide interventions, which need further testing, to improve clinical outcomes and quality of care for chronic conditions in LMICs.
Acknowledgments
The corresponding author is supported by a scholarship from the Institute of Tropical Medicine, Antwerp, Belgium.
Footnotes
Handling editor: Dr Kerry Scott
Contributors: The study was conceptualised by DL and ND. Data extraction was done by DL. All authors made substantial contributions to the analysis and interpretation of data. All authors actively contributed to the writing of the manuscript and approved the final version submitted.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: There were no primary data created or analysed in this review. All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. Naghavi M, Abajobir AA, Abbafati C, et al. . Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the global burden of disease study 2016. Lancet 2017;390:1151–210. 10.1016/S0140-6736(17)32152-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO Global status report on noncommunicable diseases 2014. World Health, 2014: 176 ISBN: 9789241564854. [Google Scholar]
- 3. Nolte EE, McKee M, Caring for people with chronic conditions : a health system perspective. European Observatory on Health Systems and Policies series, 2008: XXI, 259 ISBN: 9789289042949. [Google Scholar]
- 4. World Health Organization World Health Report 2008 “Primary Health Care : Now More Than Ever”. Geneva: World Health Organization, 2008. [Google Scholar]
- 5. Kruk ME, Nigenda G, Knaul FM. Redesigning primary care to tackle the global epidemic of noncommunicable disease. Am J Public Health 2015;105:431–7. 10.2105/AJPH.2014.302392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q 1996;74:511 10.2307/3350391 [DOI] [PubMed] [Google Scholar]
- 7. Coleman K, Austin BT, Brach C, et al. . Evidence on the chronic care model in the new millennium. Health Aff 2009;28:75–85. 10.1377/hlthaff.28.1.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beaglehole R, Epping-Jordan J, Patel V, et al. . Improving the prevention and management of chronic disease in low-income and middle-income countries: a priority for primary health care. Lancet 2008;372:940–9. 10.1016/S0140-6736(08)61404-X [DOI] [PubMed] [Google Scholar]
- 9. Ku GM, Kegels G. Adapting chronic care models for diabetes care delivery in low-and-middle-income countries: a review. World J Diabetes 2015;6:566–75. 10.4239/wjd.v6.i4.566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Puchalski Ritchie LM, Khan S, Moore JE, et al. . Low- and middle-income countries face many common barriers to implementation of maternal health evidence products. J Clin Epidemiol 2016;76:229–37. 10.1016/j.jclinepi.2016.02.017 [DOI] [PubMed] [Google Scholar]
- 11. Carroll C, Booth A, Leaviss J, et al. . “Best fit” framework synthesis: refining the method. BMC Med Res Methodol 2013;13:1–16. 10.1186/1471-2288-13-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Booth AA, Noyes J, Flemming K, 2016. Guidance on choosing qualitative evidence synthesis methods for us in health technology assessments of complex interventions. Available from: https://www.researchgate.net/profile/Andrew_Booth/publication/294581344_Guidance_on_choosing_qualitative_evidence_synthesis_methods_for_use_in_health_technology_assessments_of_complex_interventions/links/56c21c4208ae44da37ff582c.pdf
- 13. Booth A, Carroll C. How to build up the actionable knowledge base: the role of 'best fit' framework synthesis for studies of improvement in healthcare. BMJ Qual Saf 2015;24:700–8. 10.1136/bmjqs-2014-003642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carroll C, Booth A, Cooper K. A worked example of "best fit" framework synthesis: a systematic review of views concerning the taking of some potential chemopreventive agents. BMC Med Res Methodol 2011;11 10.1186/1471-2288-11-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barnett-Page E, Thomas J. Methods for the synthesis of qualitative research: a critical review. BMC Med Res Methodol 2009;9:59 10.1186/1471-2288-9-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dixon-Woods M. Using framework-based synthesis for conducting reviews of qualitative studies. BMC Med 2011;9 10.1186/1741-7015-9-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Singh D, Ham C, 2006. Improving care for people with long-term conditions. Available from: http://www.birmingham.ac.uk/Documents/college-social-sciences/social-policy/HSMC/research/long-term-conditions.pdf
- 18. Grover A, Joshi A. An overview of chronic disease models: a systematic literature review. Glob J Health Sci 2014;7:210–27. 10.5539/gjhs.v7n2p210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. WHO , 2002. Innovative care for chronic conditions: building blocks for action. Available from: http://www.who.int/chp/knowledge/publications/icccreport/en/ [Accessed 20 Aug 2013].
- 20. Davy C, Bleasel J, Liu H, et al. . Effectiveness of chronic care models: opportunities for improving healthcare practice and health outcomes: a systematic review. BMC Health Serv Res 2015;15:1–11. 10.1186/s12913-015-0854-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Innovation , 2012. MC for HC. Improving chronic illness care. Available from: http://www.improvingchroniccare.org/index.php?p=The_Chronic_Care_Model&s=2 [Accessed 7 Jan 2016].
- 22. Wagner EH, Austin BT, Davis C, et al. . Improving chronic illness care: translating evidence into action. Health Aff 2001;20:64–78. 10.1377/hlthaff.20.6.64 [DOI] [PubMed] [Google Scholar]
- 23. Fantom NJ, Serajuddin U, 2017. The World Bank's classification of countries by income (English). Policy Research. Working paper no. WPS 7528. Available from: http://documents.worldbank.org/curated/en/408581467988942234/The-World-Banks-classification-of-countries-by-income
- 24. CASP , 1994. Part of better value healthcare Ltd. Available from: www.casp-uk.net
- 25. Tapia-Conyer R, Saucedo-Martinez R, Mujica-Rosales R, et al. . Enablers and inhibitors of the implementation of the Casalud Model, a Mexican innovative healthcare model for non-communicable disease prevention and control. Health Res Policy Syst 2016;14:52 10.1186/s12961-016-0125-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chimeddamba O, Ayton D, Bazarragchaa N, et al. . The adoption of roles by primary care providers during implementation of the new chronic disease guidelines in urban Mongolia: A qualitative study. Int J Environ Res Public Health 2016;13 10.3390/ijerph13040407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Odusola AO, Stronks K, Hendriks ME, et al. . Enablers and barriers for implementing high-quality hypertension care in a rural primary care setting in Nigeria: perspectives of primary care staff and health insurance managers. Glob Health Action 2016;9 10.3402/gha.v9.29041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alberti H, Boudriga N, Nabli M. Primary care management of diabetes in a low/middle income country: a multi-method, qualitative study of barriers and facilitators to care. BMC Fam Pract 2007;8 10.1186/1471-2296-8-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bhojani U, Devedasan N, Mishra A, et al. . Health system challenges in organizing quality diabetes care for urban poor in South India. PLoS One 2014;9 10.1371/journal.pone.0106522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maimela E, Van Geertruyden JP, Alberts M, et al. . The perceptions and perspectives of patients and health care providers on chronic diseases management in rural South Africa: a qualitative study. BMC Health Serv Res 2015;15 10.1186/s12913-015-0812-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bhojani U, Mishra A, Amruthavalli S, et al. . Constraints faced by urban poor in managing diabetes care: patients' perspectives from South India. Glob Health Action 2013;6 10.3402/gha.v6i0.22258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lewis CP, Newell JN. Patients' perspectives of care for type 2 diabetes in Bangladesh -a qualitative study. BMC Public Health 2014;14 10.1186/1471-2458-14-737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shima R, Farizah MH, Majid HA. A qualitative study on hypertensive care behavior in primary health care settings in Malaysia. Patient Prefer Adherence 2014;8:1597–609. 10.2147/PPA.S69680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tlili F, Tinsa F, Skhiri A, et al. . Living with diabetes and hypertension in Tunisia: popular perspectives on biomedical treatment. Int J Public Health 2015;60(Suppl 1):31–7. 10.1007/s00038-014-0572-8 [DOI] [PubMed] [Google Scholar]
- 35. Murphy K, Chuma T, Mathews C, et al. . A qualitative study of the experiences of care and motivation for effective self-management among diabetic and hypertensive patients attending public sector primary health care services in South Africa. BMC Health Serv Res 2015;15 10.1186/s12913-015-0969-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Naanyu V, Vedanthan R, Kamano JH, et al. . Barriers influencing linkage to hypertension care in Kenya: qualitative analysis from the LARK hypertension study. J Gen Intern Med 2016;31:304–14. 10.1007/s11606-015-3566-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yan LD, Chirwa C, Chi BH, et al. . Hypertension management in rural primary care facilities in Zambia: a mixed methods study. BMC Health Serv Res 2017;17:111 10.1186/s12913-017-2063-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Legido-Quigley H, Camacho Lopez PA, Balabanova D, et al. . Patients' knowledge, attitudes, behaviour and health care experiences on the prevention, detection, management and control of hypertension in Colombia: a qualitative study. PLoS One 2015;10:e0122112 10.1371/journal.pone.0122112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fort MP, Alvarado-Molina N, Peña L, et al. . Barriers and facilitating factors for disease self-management: a qualitative analysis of perceptions of patients receiving care for type 2 diabetes and/or hypertension in San José, Costa Rica and Tuxtla Gutiérrez, Mexico. BMC Fam Pract 2013;14:131–9. 10.1186/1471-2296-14-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yellapa V, Devadasan N, Krumeich A, et al. . How patients navigate the diagnostic ecosystem in a fragmented health system: a qualitative study from India. Glob Health Action 2017;10 10.1080/16549716.2017.1350452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Risso-Gill I, Balabanova D, Majid F, et al. . Understanding the modifiable health systems barriers to hypertension management in Malaysia: a multi-method health systems appraisal approach. BMC Health Serv Res 2015;15 10.1186/s12913-015-0916-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Magadzire BP, Mathole T, Ward K. Reasons for missed appointments linked to a public-sector intervention targeting patients with stable chronic conditions in South Africa: results from in-depth interviews and a retrospective review of medical records. BMC Fam Pract 2017;18 10.1186/s12875-017-0655-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Salci MA, Meirelles BH, Silva DM. Primary care for diabetes mellitus patients from the perspective of the care model for chronic conditions. Rev Lat Am Enfermagem 2017;25:e2882 10.1590/1518-8345.1474.2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chimeddamba O, Peeters A, Ayton D, et al. . Implementation of clinical guidelines on diabetes and hypertension in urban Mongolia: a qualitative study of primary care providers' perspectives and experiences. Implement Sci 2015;10:112 10.1186/s13012-015-0307-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jacobs B, Men C, Bigdeli M, et al. . Limited understanding, limited services, limited resources: patients' experiences with managing hypertension and diabetes in Cambodia. BMJ Glob Health 2017;2 e000235 10.1136/bmjgh-2016-000235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mendenhall E, Norris SA. Diabetes care among urban women in Soweto, South Africa: a qualitative study. BMC Public Health 2015;15 10.1186/s12889-015-2615-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mendis S, Al Bashir I, Dissanayake L, et al. . Gaps in capacity in primary care in low-resource settings for implementation of essential noncommunicable disease interventions. Int J Hypertens 2012;2012:1–7. 10.1155/2012/584041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Samb B, Desai N, Nishtar S, et al. . Prevention and management of chronic disease: a litmus test for health-systems strengthening in low-income and middle-income countries. The Lancet 2010;376:1785–97. 10.1016/S0140-6736(10)61353-0 [DOI] [PubMed] [Google Scholar]
- 49. Renders CM, Valk GD, Griffin SJ, et al. . Interventions to improve the management of diabetes in primary care, outpatient, and community settings: a systematic review. Diabetes Care 2001;24:1821–33. 10.2337/diacare.24.10.1821 [DOI] [PubMed] [Google Scholar]
- 50. Samb B, Desai N, Nishtar S, et al. . Prevention and management of chronic disease: a litmus test for health-systems strengthening in low-income and middle-income countries. Lancet 2010;376:1785–97. 10.1016/S0140-6736(10)61353-0 [DOI] [PubMed] [Google Scholar]
- 51. Kane J, Landes M, Carroll C, et al. . A systematic review of primary care models for non-communicable disease interventions in Sub-Saharan Africa. BMC Fam Pract 2017;18:1–12. 10.1186/s12875-017-0613-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Esterson YB, Carey M, Piette JD, et al. . A systematic review of innovative diabetes care models in low-and middle-income countries (LMICs). J Health Care Poor Underserved 2014;25:72–93. 10.1353/hpu.2014.0037 [DOI] [PubMed] [Google Scholar]
- 53. Macinko J, Starfield B, Erinosho T. The impact of primary healthcare on population health in low- and middle-income countries. J Ambul Care Manage 2009;32:150–71. 10.1097/JAC.0b013e3181994221 [DOI] [PubMed] [Google Scholar]
- 54. Larson E, Leslie HH, Kruk ME. The determinants and outcomes of good provider communication: a cross-sectional study in seven African countries. BMJ Open 2017;7 10.1136/bmjopen-2016-014888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gilson L. Health systems and institutions : Smith R, Hanson K, Health systems in low-and middle-income countries: an economic and policy perspective. Oxford, UK: Oxford University Press, 2011. ISBN: 9780199566761. [Google Scholar]
- 56. Guanais FC, Macinko J. The health effects of decentralizing primary care in Brazil. Health Aff 2009;28:1127–35. 10.1377/hlthaff.28.4.1127 [DOI] [PubMed] [Google Scholar]
- 57. Joshi R, Jan S, Wu Y, et al. . Global inequalities in access to cardiovascular health care: our greatest challenge. J Am Coll Cardiol 2008;52:1817–25. 10.1016/j.jacc.2008.08.049 [DOI] [PubMed] [Google Scholar]
- 58. Wirtz V, Kaplan WA, Téllez YS-A, 2011. Affordable, quality, long-term care and pharmacotherapy of chronic diseases: a framework for low and middle income countries. Available from: http://www.who.int/entity/alliance-hpsr/projects/alliancehpsr_atm_chronicdiseases_mexico_wirtz.pdf
- 59. Thi Thuy Nga N, Thi My Anh B, Nguyen Ngoc N, et al. . Capacity of Commune Health Stations in Chi Linh District, Hai Duong Province, for Prevention and Control of Noncommunicable Diseases. Asia Pac J Public Health 2017;29(5_suppl) 94S–101. 10.1177/1010539517717020 [DOI] [PubMed] [Google Scholar]
- 60. Lopes Ibanez-Gonzalez D, Mendenhall E, Norris SA. A mixed methods exploration of patterns of healthcare utilization of urban women with non-communicable disease in South Africa. BMC Health Serv Res 2014;14 10.1186/s12913-014-0528-y [DOI] [PMC free article] [PubMed] [Google Scholar]