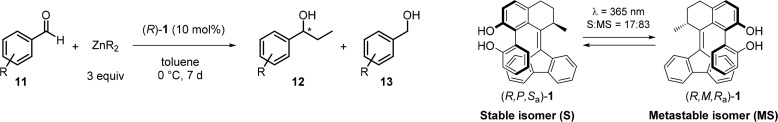
Table 1. Dynamic Enantioselective Addition of Organozinc to Aromatic Aldehydes with (R)-1.
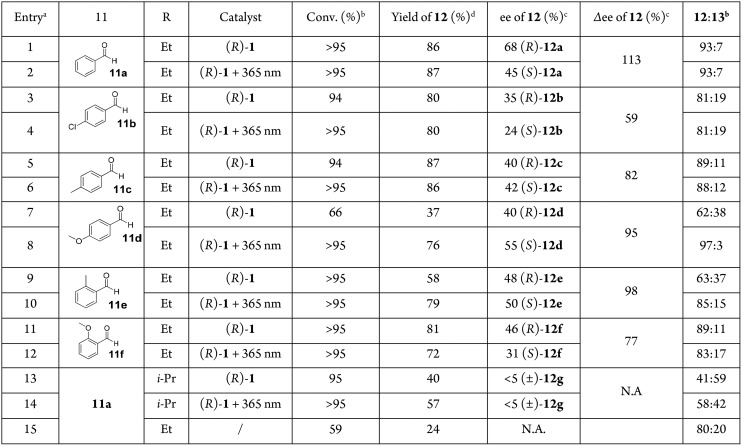
General reaction conditions: 0.0125 mmol of (R,P,Sa/Ra)-1 in 0.5 mL of dry toluene at 0 °C; 0.375 mmol of R2Zn (Et2Zn, 1.0 M in hexane; i-Pr2Zn, 1.0 M in toluene) added dropwise and stirred over 10 min; 0.125 mmol of 11 added to the mixture. Reaction mixture stirred for 7 d at 0 °C. Reaction with irradiated mixture of (R)-1: 0.00125 mmol of (R,P,Sa/Ra)-1 in 15 mL of dry, degassed Et2O, irradiated with UV light (365 nm) for 30 min until the PSS was reached (S:MS = 17:83). PSS ratio determined by chiral HPLC analysis. Reaction procedure follows as described above.
Determined by 1H NMR analysis of crude.
Determined by chiral GC or chiral HPLC analysis of isolated product.
Isolated yield. Abbreviations: N.A., Not Applicable.