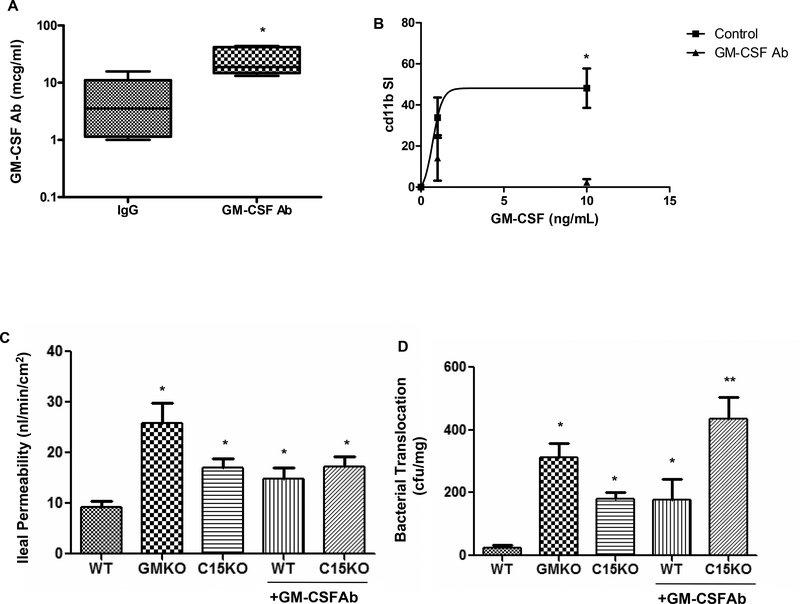
Figure 5. GM CSF Ab Administration and Barrier Function in Mice.
A) GM-CSF antibody (50 mcg IP) or isotype control were administered to wild type mice and serum was obtained two weeks later. The circulating level of GM-CSF Ab (mcg/mL) was determined by ELISA (n = 6 per group). *p<0.05 vs. IgG treated control. B) GM-CSF antibody (GM-CSF Ab, 50 mcg IP) or isotype control (IgG) were administered to wild type mice. Two weeks later, circulating leukocytes were obtained and stimulated with GM-CSF (0, 1, or 10 ng/mL) in whole blood samples and cell surface cd11b abundance was determined by flow cytometry. The cd11b stimulation index was calculated and is shown (n = 6 per group). *p<0.05 vs. GM-CSF Ab treated group at GM-CSF dose of 10 ng/mL. C) Ileal para-cellular permeability to FITC-dextran were determined using the everted gut sac method in mice fed regular chow, with and without GM-CSF Ab pre-treatment for two weeks as shown (n = 10–12 per group). *p<0.05 versus WT on regular chow. D) Bacterial translocation to draining mesenteric lymph nodes was determined in mice fed regular chow, with and without GM-CSF Ab pre-treatment for two weeks as shown (n = 10–12 per group). *p<0.05 versus WT on regular chow, **p<0.05 vs C15KO on regular chow. WT: wild type, GMKO: gm-csf deficient, C15KO: card15 deficient, GM-CSF Ab: received neutralizing GM-CSF antibody, 50 mcg IP. Data are shown as the A) median (range) or B-D) mean (SEM).