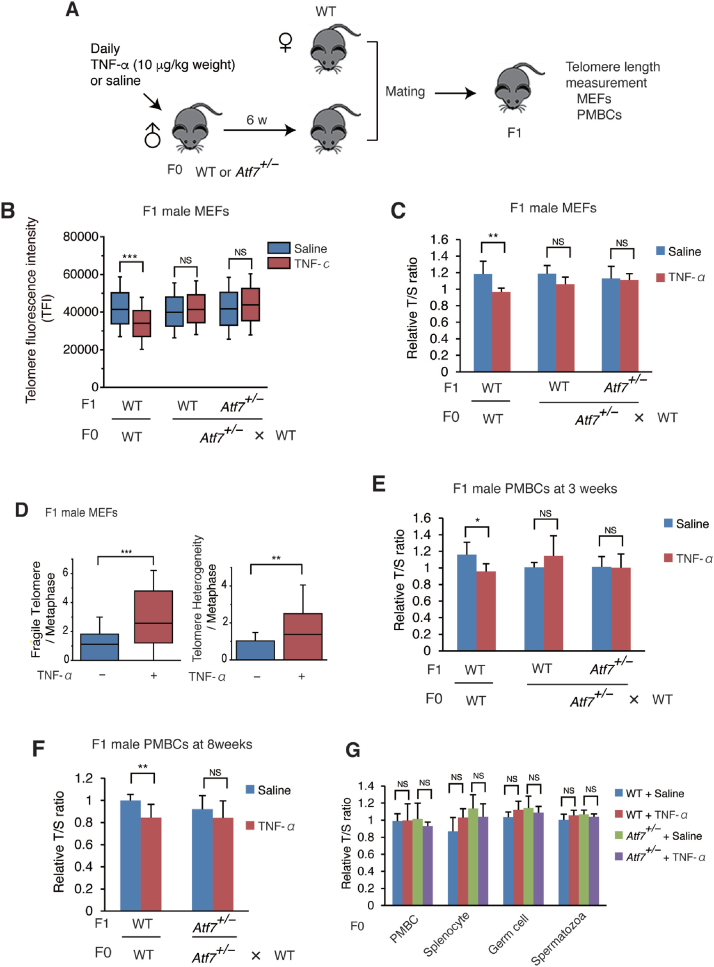
Figure 1.
Paternal TNF-α exposure induces telomere shortening in the male offspring via ATF7, but not in paternal germ cells. (A) Scheme of experimental procedures. Eight-week-old male mice (F0) were daily administered with TNF-α (10 μg/kg weight) or saline for 6 weeks, and then mated with WT female mice. Telomere length of the offspring (F1) was measured. (B, C) Paternal TNF-α treatment induces telomere shortening in MEFs from male offspring. Wide-type (WT) or Atf7+/– 8-week-old male mice (F0) (n = 3) were treated daily with TNF-α (10 μg/kg weight) or saline for 6 weeks and then mated with WT female mice (n = 3). MEFs (n = 3, three independent MEFs from three independent pregnant mice) were prepared from WT or Atf7+/– offspring (F1) for measurement of telomere length by Q-FISH (B). Raw data are shown in Supplementary Figure S1C. Middle lines in the colored boxes indicate medians; top and bottom edges, 25th and 75th percentiles; and whiskers, 10th and 90th percentiles. ***P < 0.001; NS, not significant. Telomere length in MEFs (n = 7, 6; 4, 9; 3, 5 for each group from three three independent pregnant mice) from the offspring (F1) were measured by Q-PCR (C). Relative telomere length, expressed as the T/S ratio, is shown ± SD. **P < 0.01; ***P < 0.001; NS, not significant. (D) Paternal TNF-α exposure increases the frequency of telomere fragility and telomere length heterogeneity in MEFs of male offspring. WT male offspring MEFs (n = 3, three independent MEFs from three independent pregnant mice) were prepared as described above, and the frequency of telomere fragility and telomere length heterogeneity were examined. Box plots show the abnormal telomere number per methaphase (n = 26, 31; for each group from three independent male F0 mice). Middle lines in the coloured boxes indicate medians; top and bottom edges, 25th and 75th percentiles; and whiskers, 10th and 90th percentiles. **P < 0.01; ***P < 0.001. Raw images are shown in Supplementary Figure S1D. (E, F) Paternal TNF-α exposure induces telomere shortening in peripheral blood mononuclear cells (PMBCs) from male offspring. Paternal mice were treated as above. PMBCs (n = 6, 6; 6, 5; 6, 5; n = 6, 4; 5, 5 for each group from mice generated from three independent pregnant mice, in e and f, respectively) were prepared from the 3- (E) or 8-week-old (F) male pups (F1), and telomere length was determined by Q-PCR as above. *P < 0.05; **P < 0.01; NS, not significant. (G) TNF-α exposure condition used did not induce telomere shortening in various tissues of F0 male mice. WT and Atf7+/– (n = 3) male F0 mice were treated with TNF-α or saline for 6 weeks as described above. Telomere length of PMBC, splenocytes, testicular germ cells and spermatozoa from F0 mice (n = 3) were determined by Q-PCR as above.