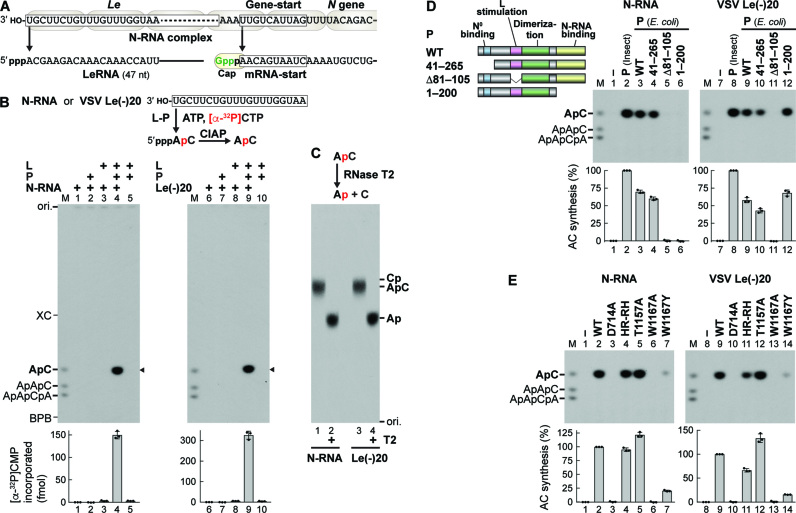
Figure 3.
The tryptophan residue in the loop of VSV L is required for terminal de novo initiation of transcription. (A) VSV LeRNA and capped N mRNA are synthesized from the 3′-terminal Le promoter and internal gene-start sequence, respectively. (B) In vitro transcription was performed with ATP, [α-32P]CTP, L, P, and/or template [N-RNA or an oligo-RNA with the VSV Le promoter sequence, VSV Le(–)20]. Calf intestine alkaline phosphatase (CIAP)-resistant RNA products were analyzed by 20% urea-PAGE followed by autoradiography. The positions of 5′-hydroxyl oligo-RNA markers (M lane), xylene cyanol FF (XC), and bromophenol blue (BPB) are shown. The graphs indicate amounts of [α-32P]CMP incorporated into RNAs (marked by arrowheads). (C) CIAP-resistant RNA products (from N-RNA or Le(–)20) were incubated with or without RNase T2, and then analyzed along with internal markers (ApC, Cp, or Ap) by PEI-cellulose TLC. (D) Schematic structures of VSV P and its deletion mutants are shown with the indicated functional domains. In vitro AC synthesis was performed with L, P (from insect cells or E. coli, WT or mutant), and template [N-RNA or VSV Le(–)20]. The graphs show relative activities of P proteins, where radioactivities of AC synthesized without (lanes 1 and 7) and with (lanes 2 and 8) WT P (insect) were set to 0 and 100%, respectively. (E) In vitro AC synthesis was performed with L (WT or mutant), P (insect), and template [N-RNA or VSV Le(–)20]. Relative AC synthesis activities of L mutants were expressed as percentages of the WT activities. The dot-plots, columns, and error bars represent the individual values, means, and standard deviations, respectively (n = 3).