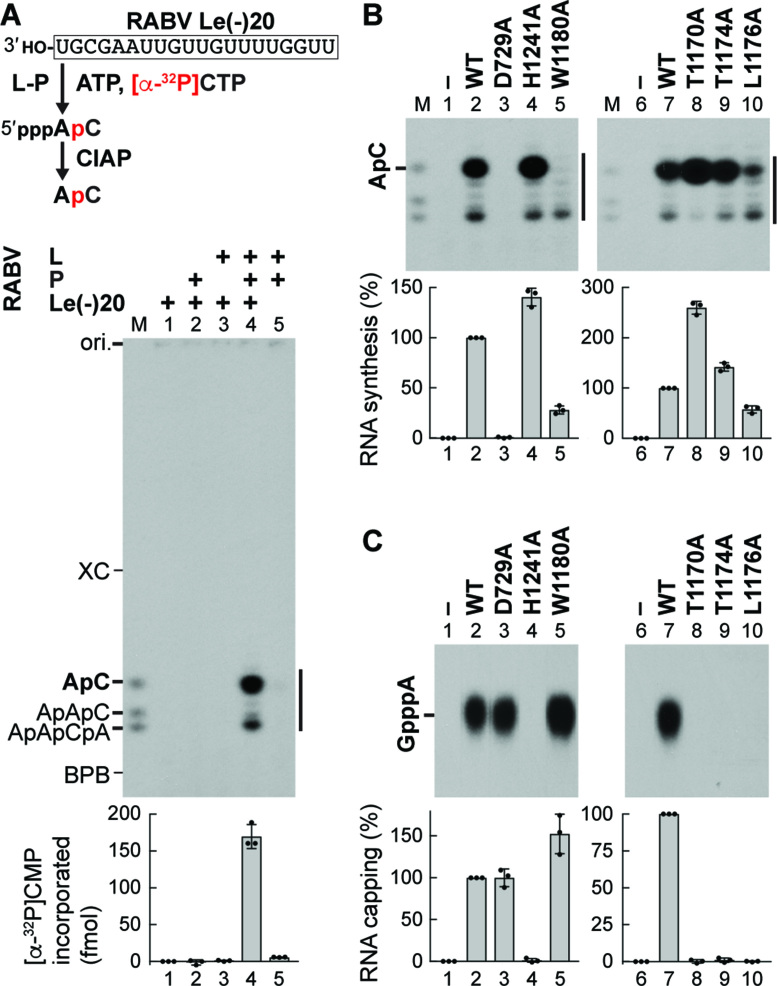
Figure 4.
A putative loop structure in the PRNTase domain of RABV L plays dual roles in RNA synthesis and capping. (A) In vitro transcription was performed with ATP, [α-32P]CTP, RABV L, P and/or an oligo-RNA with the RABV Le promoter sequence [RABV Le(–)20]. Transcripts were analyzed as in Figure 3B. The graph indicates amounts of [α-32P]CMP incorporated into RNAs (marked by the vertical line). (B, C) WT and mutant RABV L proteins were subjected to in vitro AC synthesis with P and RABV Le(–)20 (B) or capping with pppAACAC and [α-32P]GDP (C). In panel C, nuclease P1 and CIAP-resistant products were analyzed along with the standard GpppA cap by PEI-cellulose TLC. Relative RNA synthesis and capping activities of RABV L mutants were expressed as percentages of the WT activities. The dot-plots, columns, and error bars represent the individual normalized values, means, and standard deviations, respectively (n = 3).