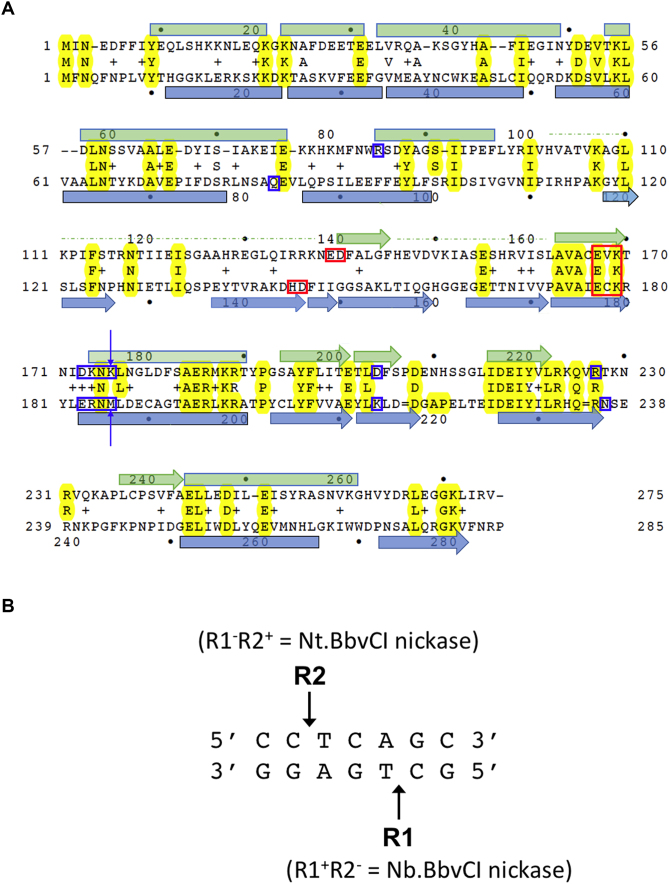
Figure 1.
Structure-based sequence alignment of R1 and R2 subunits of BbvCI restriction endonuclease. (A) Structure-based amino acid sequence alignment of the BbvCI subunits. Residues that form the PD-(D/E)XK catalytic site motifs are highlighted with red boxes. Residues postulated to be responsible for target-recognition are highlighted with blue boxes. The two residues mutated individually and in combination that alter readout of the central basepair of the target site (Figure 10) are indicated with arrows. (B)The seven base pair non-palindromic recognition site of BbvCI, and the positions of strand-cleavage catalyzed by the two subunits.