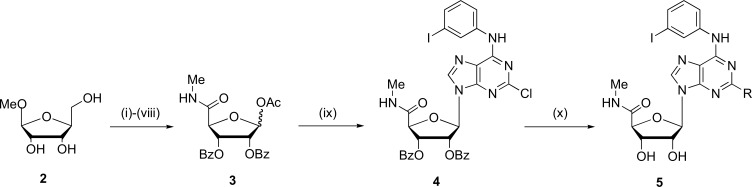
Fig. (3).
General synthesis of 2-substitution of N6-benzyladenosine- 5’-uronamides (5). Reagents and conditions: (i) TBDPSiCl, DMAP, DMF, r.t.; (ii) Bz2O, Py; n-Bu4NF, THF; (iv) RuO2, NaIO4, CHCl3:CH3CN:H2O (2:2:3); (v) EDAC, DMAP, MeOH; (vi) MeNH2, THF, 75ºC; (vii) BzCl, Py–CH2Cl2; (viii) Ac2O, H2SO4, AcOH; (ix) TMSOTf, (1,2)-dichloroethane; reflux.9-silylated adenine derivative; (x) NH3/MeOH, nucleophile. (Source: Modified from Kim et al., [46]).