Short abstract
Background
Chronic cough in children is a diagnostic challenge.
Objective
To discover the utility of nasal dipsticks and polymerase chain reaction (PCR)-DNA analysis in differentiating bacterial sinusitis from other causes of chronic cough and identifying pathogens from the nasal cavity.
Method
We recruited 22 patients under 15 years of age with cough lasting longer than 4 weeks (group 1), 7 controls with allergic rhinitis (group 2), and 10 controls without respiratory symptoms (group 3). Based on symptoms, the results of nasal secretion assays, and nasal endoscopy, a diagnosis of clinical bacterial sinusitis was made. We identified potential pathogens by quantitative PCR of nasal secretions.
Results
Group 1A (cough with clinical bacterial sinusitis n = 10): Eight (80%) patients had bacterial sinusitis associated with dominant potential pathogenic bacteria (PPB): Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Group 1B (cough without clinical bacterial sinusitis n = 12): None had dominant PPB. Group 2 (allergic rhinitis n = 7): None had dominant PPB. Group 3 (asymptomatic n = 10): None had dominant PPB. Twenty to 57% of all groups were colonized with Staphylococcus aureus. Fifty to 70% were colonized with Staphylococcus epidermidis, Corynebacterium pseudodiphtheriticum, and Dolosigranulum pigrum.
Conclusion
In children with chronic cough, clinicians can utilize a simple and inexpensive nasal secretion dipstick assay for rapid diagnosis of sinusitis and identify PPB by DNA-PCR test for specific antibiotic treatment.
Keywords: chronic cough, PCR-DNA, nasal secretion dipstick test, potential pathogenic bacteria, potential nonpathogenic bacteria, chronic sinusitis, acute sinusitis, subacute sinusitis, microbes
Introduction
Chronic cough is defined as daily cough lasting 4 weeks or longer.1 In children under 15 years of age, the causes of chronic cough include congenital defects, asthma, foreign body aspiration, infections, allergic diseases, gastro-esophageal reflux, tumors, and other rare causes.2 Among these, the most common etiologies include asthma, upper airway cough syndrome, protracted bacterial bronchitis, and nonspecific cough.3,4 The diagnosis of asthma or cough variant asthma can be made on the basis of NAEPP EPR-3 criteria and response to a bronchodilator.5 Upper airway cough syndrome is a common entity,3,4 most likely triggered by postnasal drip due to either allergic and nonallergic rhinitis or bacterial and nonbacterial sinusitis. Protracted bacterial bronchitis is now recognized as one of the most common causes of chronic wet cough, especially in young children under 5 years of age.6,7
Once asthma and other conditions listed above are ruled out, upper airway cough syndrome or protracted bacterial bronchitis need to be considered as the most likely diagnoses. In approaching these clinical entities, our goal was to promptly detect the patients with bacterial sinusitis and the involved pathogens and to treat them with appropriate antibiotics. To this end, we decided to employ a novel method described by Huang and Small,8 who reported that nasal secretion dipstick assay was highly correlated with sinus imaging studies and could be utilized for the diagnosis of sinusitis. We employed this simple approach for obvious reasons: speed, cost-effectiveness, and avoidance of radiation risk associated with sinus imaging studies. We also sought an equally simple and rapid method for identification of the pathogens for the suspected sinusitis. Positive findings in imaging studies of sinuses such as opacities and thickened lining indicate the presence of inflammation, possibly infection, but may have poor correlation with clinical disease9,10 and do not reveal any information on etiologies. Although sinus puncture would yield such information,11 this cannot be performed in most nonsurgical office settings. Studies have documented that nasal culture is a poor reflection of the microbes in the sinus cavity.12 Therefore, we analyzed the microbes from the osteomeatal area utilizing a quantitative PCR method in an attempt to detect the pathogens that may cohabit both sinus and nasal cavities.
Methods
This prospective study was conducted between 2017 and 2018 with the approval of the institutional review board and human subject committee at LA Biomed/Harbor-UCLA Medical Center. Informed consent was obtained from all patients.
Study Design and Participants
We recruited 39 children from 1 to 14 years of age composed of 3 groups.
Patients with chronic cough (group 1A and 1B)
We studied 22 children who had cough for longer than 4 weeks. Among these, 10 patients who met the clinical diagnostic criteria of bacterial sinusitis listed below were subclassified as group 1A (cough with clinical bacterial sinusitis) and the other 12 as group 1B (cough without clinical bacterial sinusitis).
Patients with allergic rhinitis (group 2)
We studied 7 patients with rhinitis and positive skin tests for respiratory allergens but without cough and lower respiratory symptoms.
Subjects without respiratory symptoms (group 3)
We studied 10 children without current or past respiratory complaints who were seen for routine physical checkups.
All patients were interviewed for pertinent history and had nasal secretion assays and quantitative PCR analysis of nasal secretions. The patients with chronic cough (group 1) and allergic rhinitis (group 2) had additional procedures: nasal endoscopy and allergy skin testing.
Clinical Diagnosis of Bacterial Sinusitis
The clinical diagnosis of bacterial sinusitis was made when a patient met 2 out of 3 criteria consisting of positive clinical history of sinusitis, nasal endoscopy, and nasal secretion score as explained below.
Clinical history
The complaint of facial pain, sinus pressure, or purulent nasal drainage was considered as positive history.
Nasal endoscopy
Jedmed flexible fiberoptic nasopharyngoscope (St. Louis, MO) was used after the application of oxymetazoline nasal spray and 2% lidocaine spray to each nostril 3 times. The presence of purulent discharge in the middle meatus or in the nasopharynx was considered to be a strong indicator for bacterial sinusitis.
Nasal secretion assay
Nasal discharge was obtained by swabbing with a wet cotton applicator 1 to 2 cm into the nasal antrum laterally and aiming at the middle meatus. The applicator tip was then smeared over a urine dip strip. The strip was scored in 4 components: protein, nitrite, pH at 60 s, and leukocytes at 120 s. The scoring was done according to the protocol published by Huang et al.8 as follows:
The total score was the sum of individual scores of protein, pH, leukocyte esterase, and nitrite. A score above 3 was considered as an indicator of bacterial sinusitis.
Analysis of Nasal Microbes
Nasal secretion samples were collected by inserting the swab into the nostrils and rubbing while rotating the swab from a depth of 2 to 3 cm and sent to MicroGen Diagnostics (Lubbock, TX) for DNA analysis of nasal microbes for rapid identification of PPB (potential pathogenic bacteria) and PPV (potential pathogenic virus).
Rapid PCR Screening and Quantification
This report, generated within 24 hours of sample collection, utilized the rapid quantitative PCR method using a Roche LightCycler 480 II instrument that detects 7 types of bacteria and the bacterial load, 1 fungus, 19 viruses, and 8 bacterial resistance genes. The bacterial primers were Haemophilus influenzae (HI), Moraxella catarrhalis, Streptococcus pyogenes, Streptococcus agalactiae, Candida albicans, Pseudomonas aeruginosa, and Staphylococcus aureus (SA). Viral primers included Human Rhinovirus/Enterovirus, Adenovirus, Coronavirus (229E, HKU1, NL63, 0C43), Human metapneumovirus, Influenza (A H1, H3, H1 2209, B), Parainfluenza Virus (1, 2, 3, 4), Respiratory Syncytial Virus, Bordetella pertussis, Chlamydophila pneumoniae, and Mycoplasma pneumoniae. Resistance genes for the following antibiotics were included vancomycin, methicillins, beta-lactams, carbapenems, macrolides, aminoglycosides, tetracyclines, and quinolones. Once the presence of bacteria was identified, relative quantity (abundance) of these was reported in percentage of total bacterial load subsequently by using NGS (next-generation sequencing) machine. The method utilized universal 16S and internal transcribed space amplicon sequencing on the Ion Torrant Personal Genome Machine. A bacterium with abundance greater than 50% was considered to be a dominant species.
Classification of Microbiome
Streptococcus pneumonia (SP), HI, and Moraxella catarrhalis (MC) were classified as PPB, and Staphylococcus epidermidis (SE), Corynebacterium pseudodiphtheriticum (CP), and Dolosigranulum pigrum (DP) were classified as PNPB. SA was classified separately. Rhinovirus and Parainfluenza Virus were classified as potentially pathogenic viruses (PPV). These classifications will be elaborated in the “Discussion” section.
Skin Testing
Allergy skin tests were employed to identify patients with allergic rhinitis (group 2). Tests were performed according to a standard procedure using 16 to 40 inhalant allergens (Stallergenes Greer; London, UK) applied to the skin by Multitest applicator (Lincoln Diagnostics; Decatur, IL). The allergens included dust mites, cat, dog, molds, and pollens of grass, trees, and weeds specific to Southern California.
Statistical Analyses
Means or medians and standard deviations or interquartile ranges were computed for continuous variables. Frequencies and percentages were compared for categorical variables. Analysis of variance was used if the outcome followed a normal distribution. The Kruskal–Wallis test was used for nonnormal continuous outcomes. Chi-square or Fisher exact tests were used to compare differences in proportions among groups. SAS 9.4 Software (Cary, NC) was used for all data analyses.
Results
As stated in the “Methods” section, subjects who met 2 out of 3 sinusitis criteria were classified as group 1A. Nasal endoscopic findings (P < .04) and nasal secretion scores (P < .0001) were highly sensitive and could have been used as the selection criteria by themselves as shown in Table 1.
Table 1.
Demographics and Clinical Characteristics.
| Group 1A: Cough w/ Bacterial Sinusitis (n = 10) | Group 1B: Cough w/o Bacterial Sinusitis (n = 12) | Group 2: Allergic Rhinitis (n = 7) | Group 3: Asymptomatic (n = 10) | Pa | |
|---|---|---|---|---|---|
| Mean age, y (range) | 4.7 (2–12) | 5.8 (3–12) | 8.0 (1–12) | 5.6 (2–9) | NA |
| Male sex, n (%) | 6 (60%) | 5 (42%) | 4 (57%) | 5 (50%) | |
| Race/ethnicity, n | |||||
| White | 4 | 5 | 4 | 3 | |
| African American | 1 | 0 | 0 | 1 | |
| Hispanic | 3 | 4 | 0 | 4 | |
| Asian | 2 | 3 | 3 | 2 | |
| Mean cough duration (wks) | 15 | 31 | 0 | 0 | |
| Positive nasal endoscopy for sinusitis, n (%) | 8 (80)b | 2 (17) | 2 (29) | NA | .004 |
| Mean nasal secretion score (range) | 5.4 (3–7)b | 1.8 (1–5) | 1.9 (0–5) | 0.7 (0–1) | <.0001 |
| Positive allergy skin test/IgE, n (%) | 5 (50) | 11 (91) | 7 (100) | NA |
P values are based on Kruskal–Wallis test.
P values are expected to be statistically significant since positive nasal endoscopy findings and nasal secretion scores were used as selection criteria to diagnose bacterial sinusitis.
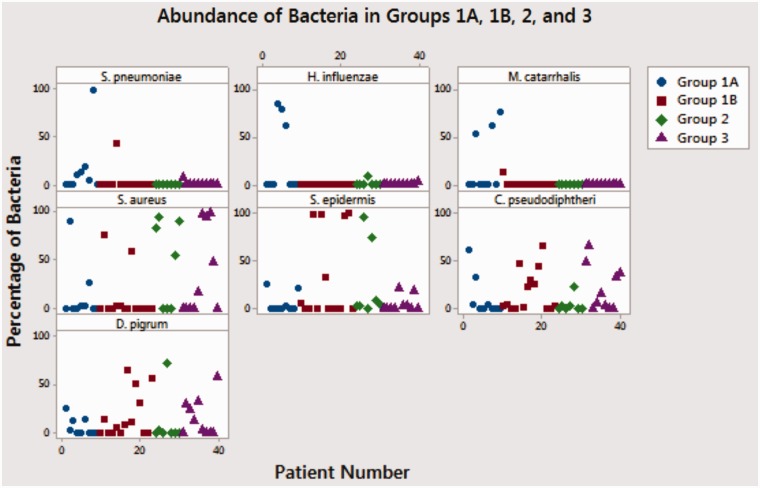
Patterns of Microbes Among Different Clinical Groups (Tables 2 and 3, and Figure 1)
Table 2.
Summary of Microbe Distribution Among Different Groups.
| Group 1A: Cough w/ Bacterial Sinusitis (n = 10) | Group 1B: Cough w/o Bacterial Sinusitis (n = 12) | Group 2: Allergic Rhinitis (n = 7) | Group 3: Asymptomatic (n = 10) | P | |
|---|---|---|---|---|---|
| Subjects with PPB (% of total) | 8 (80%)a | 2 (17%) | 1 (14%) | 1 (10%) | .002 |
| Subjects with NPPB (% of total) | 5 (50%) | 10 (83%) | 4 (57%) | 7 (70%) | .4 |
| Subjects with SA (% of total) | 2 (20%) | 2 (17%) | 4 (57%) | 4 (40%) | .3 |
| Subjects with SE (% of total) | 2 (20%) | 3 (25%) | 3 (43%) | 3 (30%) | .8 |
| Subjects with CP (% of total) | 2 (20%) | 6 (50%) | 1 (14%) | 5 (50%) | .3 |
| Subjects with DP (% of total) | 3 (30%) | 8 (75%) | 1 (14%) | 6 (60%) | .09 |
| Subjects with PPV (% of total) | 6 (60%) | 3 (25%) | 1 (14%) | 2 (20%) | .2 |
Abbreviations: CP, Corynebacterium pseudodiphtheriticum; DP, Dolosigranulum pigrum; PNPB, potentially nonpathogenic bacteria; PPB, potentially pathogenic bacteria; PPV, potentially pathogenic virus; SA, Staphylococcus aureus; SE, Staphylococcus epidermidis.
Group 1A had significantly more PPB demonstrated in their nasal cavity.
Table 3.
Number of Subjects With Dominant (≥50% of Total) of Bacteria in Each Group.
| Group 1A: Cough w/ Bacterial Sinusitis (n = 10) | Group 1B: Cough w/o Bacterial Sinusitis (n = 12) | Group 2: Allergic Rhinitis (n = 7) | Group 3: Asymptomatic (n = 10) | P for the Presence of Dominant Bacteria in Nasal Secretions | |
|---|---|---|---|---|---|
| Subjects with dominance of PPB (% of total) | 8 (80%)a | 0 (0%) | 0 (0%) | 0 (0%) | <.0001 |
| Subjects with dominance of SA (% of total) | 1 (10%) | 2 (17%) | 4 (57%) | 2 (20%) | .2 |
| Subjects with dominance of PNPB (% of total) | 1 (10%) | 3 (25%) | 2 (29%) | 1 (10%) | .7 |
Abbreviations: PNPB, potentially nonpathogenic bacteria; PPB, potentially pathogenic bacteria; SA, Staphylococcus aureus.
aOnly group 1A (cough with clinical bacterial sinusitis) demonstrated the presence of dominant bacteria in their nasal secretion samples by DNA techniques (P < .0001). Bacteria with abundance greater than 50% was considered to be a dominant species.
Figure 1.
Abundance of bacteria in groups 1A, 1B, and 3.
The group with clinical diagnosis of bacterial sinusitis (group 1A) carried PPB in their nasal cavity in higher percentage. NPPB, SA, and PPV were present in various degrees in all 3 groups, but there were no statistically significant patterns discernible.
Group 1A
Among the 10 patients who met the clinical criteria for bacterial sinusitis, 8 patients (80%) (P = .02) demonstrated the presence of PPB in their nasal samples including SP, HI, and MC. All 8 patients had one of these PPB as a dominant species (P < .0001). Six (60%) patients demonstrated the presence of PPV antigens and 4 (40%) had concomitant PPB and PPV.
Group 1B
Twelve patients were classified into a nonbacterial cough group since they did not meet the above bacterial sinusitis criteria. Two patients (17%) demonstrated the presence of PPB, but none of which were dominant. Three (25%) patients demonstrated the presence of PPV antigens and 1 (8%) had concomitant PPB and PPV.
Group 2
Among 7 patients with allergic rhinitis, 1 patient had nondominant PPB. One (14%) patient carried PPV.
Group 3
Among 10 asymptomatic controls, 1 patient (10%) had nondominant PPB. Two patients (20%) had PPV.
Clinical and Nasal Secretion Assay Response to Antibiotics
All patients in group 1A reported improvement of cough after 2 to 8 weeks of antibiotic treatment. Nasal secretion assay score also decreased below 4 in all. Patients in group 1B were symptomatically managed with varying success without the use of antibiotics.
Allergic Sensitization
All patients in group 1A, 1B, and 2 were tested for the sensitization to aeroallergens. Allergic sensitization was present in 5 out of 10 patients (50%) in group 1A and 11 out of 12 patients (91%) in group 1B.
Discussion
In children, acute sinusitis is usually defined as symptoms lasting less than 30 days, chronic sinusitis longer than 90 days, and subacute sinusitis in between these 2 ranges.13 Acute sinusitis is associated with sinus pressure, purulent nasal drainage, cough, and the presence of PPB.13,14 In chronic and subacute sinusitis, protracted cough may play a more prominent role than nasal symptoms.13,15 The pathology of subacute sinusitis may resemble acute sinusitis,14 while chronic sinusitis may involve inflammatory processes in association with various microbes including SA, especially in patients with nasal polyposis.14–16 Among group 1 patients, 9 patients had chronic and 13 patients subacute symptoms (data not shown).
Once we made the clinical diagnosis of bacterial sinusitis based on the previously mentioned criteria (group 1A), we sought to identify the predominant bacteria associated with the condition. Although asymptomatic children may be colonized by PPB in the middle meatus,17 the presence of these PPB in the nasal cavity of patients diagnosed with clinical sinusitis may reveal the pathogens. Lee et al. reported a study of 31 adults with acute sinusitis in which 61% of nasopharyngeal and 48% of middle meatal samples grew PPB and that the concordance rate between the 2 sites was 84%.18 We wanted to increase the sensitivity of microbial identification by employing DNA-based molecular diagnostics. Recent studies indicate that molecular diagnostic approaches yield much more accurate results with regard to diversity and quantities of microbes present in the sinus and nasal cavities.19–23
Once the PCR results were reported, we classified these bacteria into different categories depending on their pathogenic potential. SP, HI, and MC were classified as PPB since these have been established as predominant pathogens for acute and subacute sinusitis by previous studies.12,24 SE is reported to be a commensal bacterium in the nasal cavity.25 Although CP was characterized as pathogenic in some studies,26 it was reported to be part of natural microbiota of the nares and throat.27 DP has not been reported to cause sinusitis.28 SA was classified separately because of its presence in the nasal and sinus cavities of asymptomatic subjects29–31 and patient with sinusitis.32,33 Rhinovirus and Parainfluenza Virus were the only viruses identified and classified as potentially pathogenic viruses (PPV) since it is well known to cause upper respiratory symptoms. As seen in Tables 2 and 3, the presence of PPB as a dominant species in the nasal cavities correlated with the clinical diagnosis of bacterial sinusitis (P < .002) indicating that dominance of microbes over others is an important element of pathogenesis.34 The presence of a PPB in group 1B, 2, and 3 is not surprising because it is known to inhabit the nasal cavities of asymptomatic subjects.17 The nondominance of these PPB along with other clinical features should enable the clinicians to separate these groups from the ones with bacterial sinusitis (1A). Group 1A may represent a combination of recurrent acute, subacute, and chronic sinusitis with bacterial infections. These PPB are also reported to be the most common pathogens associated with acute otitis media33 and protracted bacterial bronchitis.6,7 It is possible that these patients may indeed have simultaneous sinusitis and protracted bacterial bronchitis since antibiotic treatment stops coughing in both entities. Such coexistence (via “one airway”) needs to be explored in the future. In group 1B, chronic cough was most likely due to postnasal drip resulting from nonbacterial pathologies such as allergy (91% of the patients had allergen sensitization as shown in Table 1), viral infection, or chronic irritation. Although SA was present as dominant species in 2 patients in this group, its pathogenic role is doubtful due to their presence in control groups 2 and 3. Staphylococci were present in the sinus aspirates of acute,29 chronic sinusitis,14,20,21 and in the nasal cavities of those with CRS with nasal polyps,16 and may play a role in the pathogenesis of sinusitis, especially in adults.32,33 But they were also shown to be present in the nasal cavities of 25% to 30% of asymptomatic subjects30 in a competing relationship with PNPB,22 and their presence may be a transient colonization rather than infection.36
The presence of PNPB in all groups (Table 2 and Figure 1) is consistent with the recent studies revealing asymptomatic cohorts also carry diverse commensal bacteria in the nasal cavities including SA, SE, DP, and CP, and they maintain competing relationship among themselves.20,22 An intriguing finding was the presence of rhinovirus in all groups including asymptomatic controls, indicating that its colonization was not necessarily associated with clinical symptoms. In group 1A, 60% of the patients carried PPV and 40% carried both PPB and PPV indicating that both viruses and bacteria were coexistent. Rhinovirus, the most common viral pathogen in our study, was shown to increase adhesion of PPB to nasal epithelial cells at least 2-fold compared to uninfected controls.37,38
Conclusion
We demonstrated, as others have, in children with chronic cough with sinusitis, microbial balance of the sinus cavity is disturbed and PPB takes the dominance. This can be detected by simple methods: dipstick assay and quantitative DNA-PCR test of the nasal secretion. Therefore, we recommend that clinicians utilize these methods as an alternative to imaging studies to make a diagnosis of bacterial sinusitis and offer a specific therapy for the pathogens in a rapid and accurate manner. Further studies are indicated to replicate these findings and refine the potential diagnostic capabilities of DNA-based methods for bacterial sinusitis and bronchitis.
Acknowledgments
The editors gratefully acknowledge the assistance of Margaret Keller, MD, Lynn Smith, MD, and Joaquin Madrenas, MD, PhD, who reviewed the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval
This study was approved by Los Angeles BioMed. Clinical trial registration: https://imedris.labiomed.org/database#30907-01.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: DNA-based analysis was provided to the authors free of charge by MicroGen Diagnostics (Lubbock, TX).
Approved by Institutional Review Board.
Statement of Informed Consent
Informed consents were obtained from all subjects who participated in this project.
Statement of Human and Animal Rights
This article does not contain any studies with animal subjects.
References
- 1.Chang AB, Oppenheimer JJ, Weinberger M, et al. Use of management pathways or algorithms in children with chronic cough: systematic reviews. Chest. 2016; 149(1):106–117. [DOI] [PubMed] [Google Scholar]
- 2.Chang AB, Robertson CF, Van Asperen PP, et al. A multicenter study on chronic cough in children: burden and etiologies based on a standardized management pathway. Chest. 2012; 142(4):943–950. [DOI] [PubMed] [Google Scholar]
- 3.Chang AB, Oppenheimer JJ, Weinberger MM, et al. Etiologies of chronic cough in pediatric cohorts: CHEST Guideline and Expert Panel Report. Chest. 2017; 152:607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asilsoy S, Bayram E, Egin H, et al. Evaluation of chronic cough in children. Chest. 2008; 134:1122–1128. [DOI] [PubMed] [Google Scholar]
- 5.National Asthma Education and Prevention Program. Guidelines for the Diagnosis and Management of Asthma: Expert Panel Report 3. National Institute of Health 2007, NIH publication 07-4051.
- 6.Marchant JM, Masters IB, Taylor SM, et al. Evaluation and outcome of young children with chronic cough. Chest. 2006; 129:1132–1141. [DOI] [PubMed] [Google Scholar]
- 7.Chang AB, Upham JW, Masters IB, et al. Protracted bacterial bronchitis: the last decade and the road ahead. Pediatr Pulmonol. 2016; 51:225–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang SW, Small PA. Rapid diagnosis of bacterial sinusitis in patients using a simple test of nasal secretions. Allergy Asthma Proc. 2008; 29(6):640–643. [DOI] [PubMed] [Google Scholar]
- 9.Chang AB, Glomb WB. Guidelines for evaluating chronic cough in pediatrics: ACCP evidence-based clinical practice guidelines. Chest. 2006; 129:260S. [DOI] [PubMed] [Google Scholar]
- 10.Shopfner CE, Rossi JO. Roentgen evaluation of the paranasal sinuses in children. Am J Roentgenol Radium Ther Nucl Med. 1973; 118(1):176. [DOI] [PubMed] [Google Scholar]
- 11.Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012; 54(8):e72–e112. [DOI] [PubMed] [Google Scholar]
- 12.Wald ER, Milmoe GJ, Brown A, et al. Acute maxillary sinusitis in children. N Engl J Med. 1981; 304(13):749–754. [DOI] [PubMed] [Google Scholar]
- 13.Wald ER, Applegate KE, Bordley C, et al. Clinical practice guideline for the diagnosis and management of acute bacterial sinusitis in children aged 1–18 years. Pediatrics. 2013; 132:e262. [DOI] [PubMed] [Google Scholar]
- 14.Wald ER. Microbiology of acute and chronic sinusitis in children. J Allergy Clin Immunol. 1992; 90:452–460. [DOI] [PubMed] [Google Scholar]
- 15.Hopp RJ, Allison J, Brooks D. Fifty years of chronic rhinosinusitis in children: the accepted, the unknown, and thoughts for the future. Pediatr Allergy Immunol Pulmonol. 2016; 29:61–67. [DOI] [PubMed] [Google Scholar]
- 16.Van Zele T, Gevaert P, Watelet JB, et al. Staphylococcus aureus colonization and IgE antibody formation to enterotoxins is increased in nasal polyposis. J Allergy Clin Immunol. 2004; 114(4):98–983. [DOI] [PubMed] [Google Scholar]
- 17.Gordts F, Abu Nasser I, Clement PA, et al. Bacteriology of the middle meatus in children. Int J Pediatr Otorhinolaryngol. 1999; 48(2):163–167. [DOI] [PubMed] [Google Scholar]
- 18.Lee S, Woodbury K, Ferguson BJ. Use of nasopharyngeal culture to determine appropriateness of antibiotic therapy in acute bacterial rhinosinusitis. In Forum Allergy Rhinology. 2013; 4:272–275. [DOI] [PubMed] [Google Scholar]
- 19.Kreft R, Costerton W, Ehrlich G. PCR is changing clinical diagnostics. Microbe. 2013; 8(1):15–20. [Google Scholar]
- 20.Feazel LM, Robertson CE, Ramakrishnan VR, Frank DN. Microbiome complexity and Staphylococcus aureus in chronic rhinosinusitis. Laryngoscope. 2012; 122(2):467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hauser LJ, Feazel LM, Ir D, et al. Sinus culture poorly predicts resident microbiota. Int Forum Allergy Rhinol. 2015; 5(1):3–9. [DOI] [PubMed] [Google Scholar]
- 22.Yan M, Pamp SJ, Fukuyama J, et al. Nasal microenvironments and interspecific interactions influence nasal microbiota complexity and S. aureus carriage. Cell Host Microbe. 2013; 14(6):631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahdavinia M, Keshavarzian A, Tobin MC. A comprehensive review of the nasal microbiome in chronic rhinosinusitis. Clin Exp Allergy. 2016; 46(1):21–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gwaltney JM, Jr, Scheld WM, Sande MA, et al. The microbial etiology and antimicrobial therapy of adults with acute community-acquired sinusitis: a fifteen-year experience at the University of Virginia and review of other selected studies. J Allergy Clin Immunol. 1992; 90(3 Pt 2):457–462. [DOI] [PubMed] [Google Scholar]
- 25.Iwase T, Uehara Y, Shinji H, et al. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature. 2010; 465:346–349. [DOI] [PubMed] [Google Scholar]
- 26.Abreu NA, Nagalingam NA, Song Y, et al. Sinus microbiome diversity depletion and Corynebacterium tuberculostearicum enrichment mediates rhinosinusitis. Sci Transl Med. 2012; 4(151):151ra124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Funke G, von Graevenitz A, Clarridge JE, III, et al. Clinical microbiology of coryneform bacteria. Clin Microbiol Rev. 1997; 10:125–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lécuyer H, Audibert J, Bobigny A, et al. Gram-positive bacterium from the genus of Dolosigranulum. Dolosigranulum pigrum can cause infections in the upper respiratory tract and nosocomial pneumonia and septicemia. Clin Microbiol. 2007; 45(10):3474–3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wald ER. Staphylococcus aureus: is it a pathogen of acute bacterial sinusitis in children and adults? Clin Infect Dis. 2012; 54(6):826–831. [DOI] [PubMed] [Google Scholar]
- 30.Lina G, Boutite F, Tristan A, et al. Bacterial competition for human nasal cavity colonization: role of Staphylococcal agr alleles. Appl Environ Microbiol. 2003; 69(1):18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brook I. Aerobic and anaerobic flora of normal maxillary sinuses. Laryngoscope. 1981; 91(3):372–376. [PubMed] [Google Scholar]
- 32.Payne SC, Benninger MS. Staphylococcus aureus is a major pathogen in acute bacterial rhinosinusitis: a meta-analysis. Clin Infect Dis. 2007; 45:121–127. [DOI] [PubMed] [Google Scholar]
- 33.Orlandi RR, Kingdom TT, Hwang PH, et al. International consensus statement on allergy and rhinology: rhinosinusitis. Int Forum Allergy Rhinol. 2015; 6:S22–S209. [DOI] [PubMed] [Google Scholar]
- 34.Boase S, Foreman A, Cleland E, et al. The microbiome of chronic rhinosinusitis: culture, molecular diagnostics and biofilm detection. BMC Infect Dis. 2013; 13:210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brook I, Yocum P, Shah K. Aerobic and anaerobic bacteriology of concurrent chronic otitis media with effusion and chronic sinusitis in children. Arch Otolargyngol Head Neck Surg. 2000; 126:174–176. [DOI] [PubMed] [Google Scholar]
- 36.Van Belkum A, Verkaik NJ, de Vogel CP, et al. Reclassification of Staphylococcus aureus nasal carriage types. J Infect Dis. 2009; 199:1820–1826. [DOI] [PubMed] [Google Scholar]
- 37.Ishizuka S, Yamaya M, Suzuki T. Effects of rhinovirus infection on the adherence of Streptococcus pneumoniae to cultured human airway epithelial cells. J Infect Dis. 2003; 188:1928–1939. [DOI] [PubMed] [Google Scholar]
- 38.Wong J, Kwon H, Jang Y. Rhinovirus enhances various bacterial adhesions to nasal epithelial cells simultaneously. Laryngoscope. 2009: 119(7);1406–1411. [DOI] [PubMed] [Google Scholar]