Abstract
This data is related to the research article entitled “Germinal center humoral autoimmunity independently mediates progression of allograft vasculopathy” (Harper et al., 2016) [2]. The data presented here focuses on the humoral autoimmune response triggered by transferred allogeneic CD4 T cells and includes details on: (a) the recipient splenic germinal center (GC) response; (b) augmentation of humoral autoimmunity and accelerated heart allograft rejection following transplantation from donors primed against recipient; (c) flow cytometric analysis of donor and recipient CD4 T cells for signature markers of T follicular helper cell differentiation; (d) in vitro donor endothelial cell migration in response to column purified autoantibody from recipient sera; (e) analysis of development of humoral responses in recipients following adoptive transfer of donor CD4 T cells and; (f) the development of humoral autoimmunity in mixed haematopoietic chimeric mice.
Specifications table
| Subject area | Autoimmunity |
| More specific subject area | Transplant related autoimmunity |
| Type of data | Figures |
| How data was acquired | Microscopy (Olympus, Japan), flow cytometry [FACSCanto II flow cytometer with FACSDiva (BD Biosciences, San Jose, CA)], ELISA [FLUOstar OPTIMA plate reader (BMG Labtech, Aylesbury, U.K.)] |
| Data format | Analyzed |
| Experimental factors | Humoral autoimmune responses measured by relative antibody concentration, percentage of germinal centers, area occupied by GCs, percentage of TFHcells, number of cells |
| Experimental features | Humoral Autoimmune responses were analysed |
| Data source location | Department of Surgery, University of Cambridge, Level 9 Lab |
| Data accessibility | Data is with this article |
| Related research article | M. S. Qureshi, J. Alsughayyir, M. Chhabra, J. M. Ali, M. J. Goddard, C. Devine et al. Germinal center humoral autoimmunity mediates progression of allograft vasculopathy independently from recipient alloimmunity. J Autoimmun, 2018; In press[2] |
Value of the data
-
•
The data presented provides a guide for performing a comprehensive analysis of germinal center autoantibody responses in transplantation.
-
•
This data may help in designing strategies, such as ex-vivo normothermic donor organ perfusion, in which it is possible to modulate the donor lymphocyte fraction prior to implantation.
-
•
The data can provide a framework for future studies to delineate the role of donor lymphocytes and epitope diversification in host alloimmune responses.
1. Data
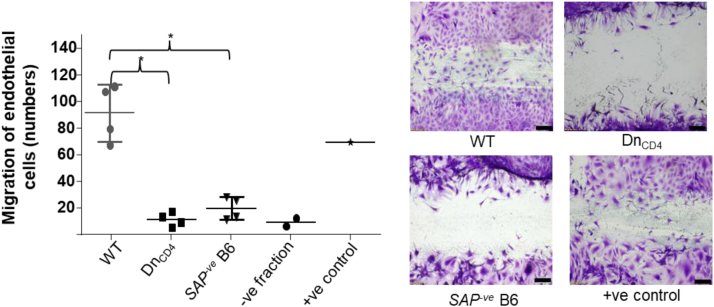
The data presented here was obtained after WT bm12 or CD4 T cell depleted bm12 donor animals either heart transplants or cells into WT B6 or T cell deficient B6 recipients. Priming of donor animals against the recipient, prior to transplantation into the same recipient strains, resulted in augmented humoral responses (Fig. 2a and b) with rapid rejection of donor allografts (Fig. 2c). Splenic GC areas were significantly higher in WT compared to Tcrbd−/− recipients (DnCD4 group) and WT recipients who received CD4 depleted donor bm12 allograft (RcCD4) (Fig. 3). Adoptive transfer of bm12 CD4 T cells into congenic CB45.1 B6 recipients demonstrated that a small percentage of recipient (CD45.1+ve) CD4 T cells acquired TFH cell signature markers compared to donor (CD45.2+ve) CD4 T cells (Fig. 4a and b) and 3.5% of recipient B cells were found to be double positive on flow cytometry for Ki67 and Bcl-6 that are characteristic of GC B cells (Fig. 4c). Adoptive transfer of bm12 CD4 T cells into Tcrbd−/− and SAP-ve B6 groups revealed short lasting autoantibodies and GCs compared to WT group (Fig. 5a, b). Furthermore in-vitro EC migration assays demonstrated significantly higher number of bm12 ECs migrated across the scratch wound line in response to (column-purified) immunoglobulin from WT recipients of bm12 heart transplants, compared to immunoglobulin from T cell deficient Tcrbd−/−(DnCD4) B6 recipients or from Sh2d1a−/− B6 recipients that are genetically deficient in SLAM-associated protein (SAP) and that do not form TFH cells (SAP-veB6, Fig. 6).
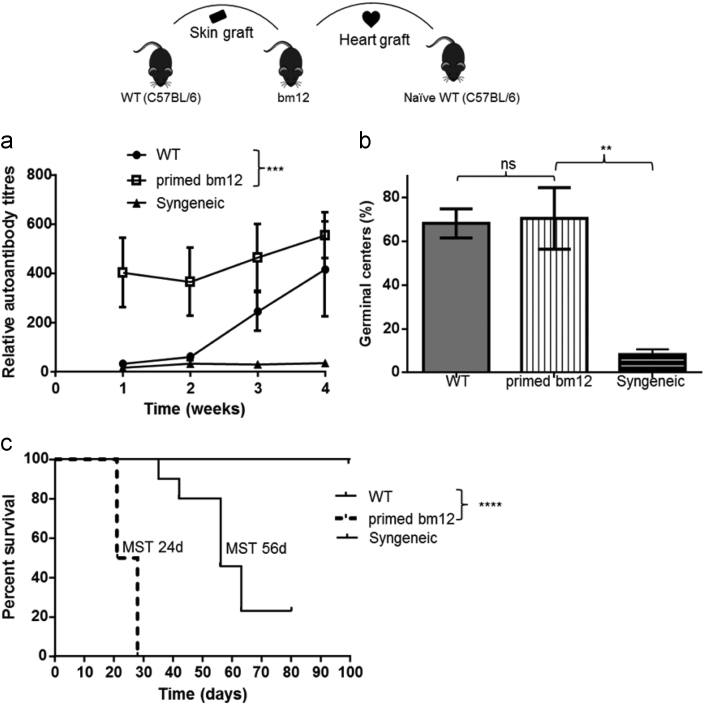
Fig. 2.
Augmented anti-nuclear autoantibody response and accelerated heart allograft rejection following transplantation from donors primed against recipient. WT.B6 mice were transplanted with hearts from bm12 donors sensitised to recipients by challenge with WT B6 skin grafts 7 weeks previously (primed bm12). Control recipient B6 mice received heart allografts from unmodified bm12 donors (WT). Anti-nuclear autoantibody titres following transplantation (a); splenic germinal center activity (percentage of secondary follicles) (b); and allograft survival (c) are shown. Included for comparison are results for B6 recipients of syngeneic B6 heart transplants. Data represent mean and SD of n = 4–10 mice per group. ns – not significant: *P < 0.05, **P < 0.01 and ***P < 0.001.
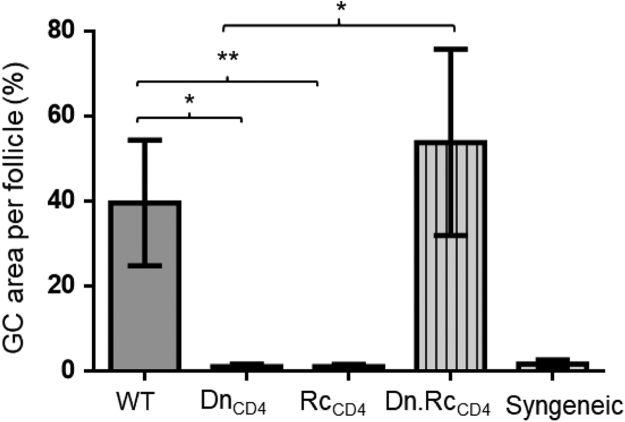
Fig. 3.
Calculation of germinal center area. Splenic GC area was significantly higher in WT compared to T cell deficient (Tcrbd−/−) recipients (DnCD4) and WT recipients who received CD4 depleted donor bm12 allograft (RcCD4). However, GC area was comparable between WT and Tcrbd−/− recipients reconstituted with WT B6 CD4 T cells at the time of transplantation of bm12 allografts (Dn.RcCD4). Splenic GC area in B6 recipients of syngeneic B6 heart grafts is included for comparison. Data represent mean and SD of n = 4–6 mice per group. *P < 0.05, **P < 0.01.
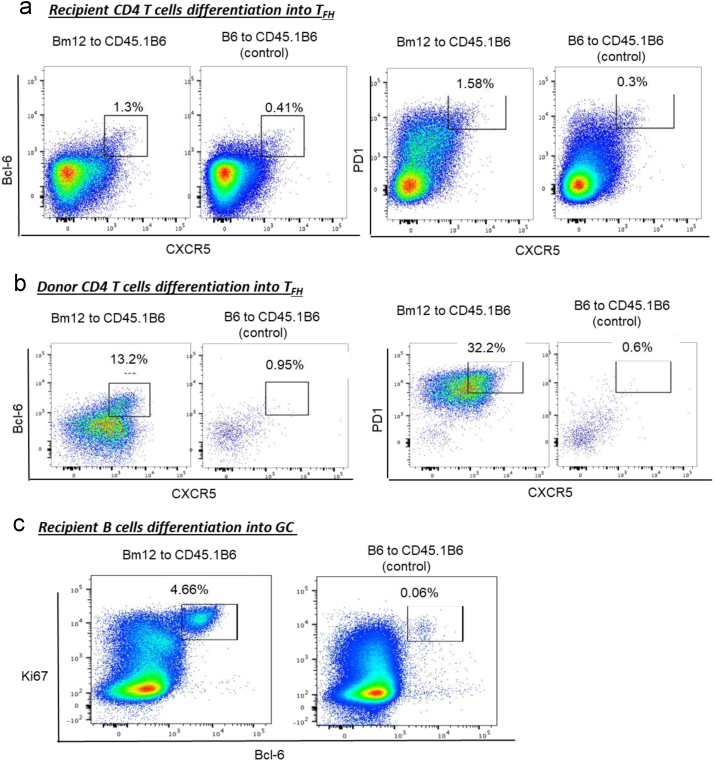
Fig. 4.
Differentiation of CD4 T cells into TFH phenotype and B cells into germinal centers on flow cytometry in WT recipients (CD45.1 B6) of bm12 CD4 T cells (CD45.2). CD45.1 B6 mice were challenged with either purified allogeneic CD45.2 bm12 CD4 T cells or CD45.2B6 CD4 T cells. 23 days later, mice were sacrificed and donor (CD45.2+ve) and recipient (CD45.2-ve) splenic CD4 T cells were characterized for TFH markers and recipient splenic B cells for GC B cell markers. Foxp3-ve, CD45.2-ve (recipient (a)) and CD45.2+ve (donor (b)) splenic CD4 T cell populations characterised by flow cytometry for acquisition of CXCR5hiBcl-6hi (left panel) or CXCR5hiPD1hi (right panel) TFH cell phenotype and recipient B220+ve B cell population (c) characterized by flow cytometry for acquisition of GC markers of Bcl-6hi and Ki67hi are shown. Data for TFH cells and GC B cells for WT recipients (CD45.1 B6) of syngeneic B6 (CD45.2 B6) CD4 T cells is included for comparison. Figures represent one of two separate experiments, with values depicting proportion of CD4 T cell and B cell population within gates.
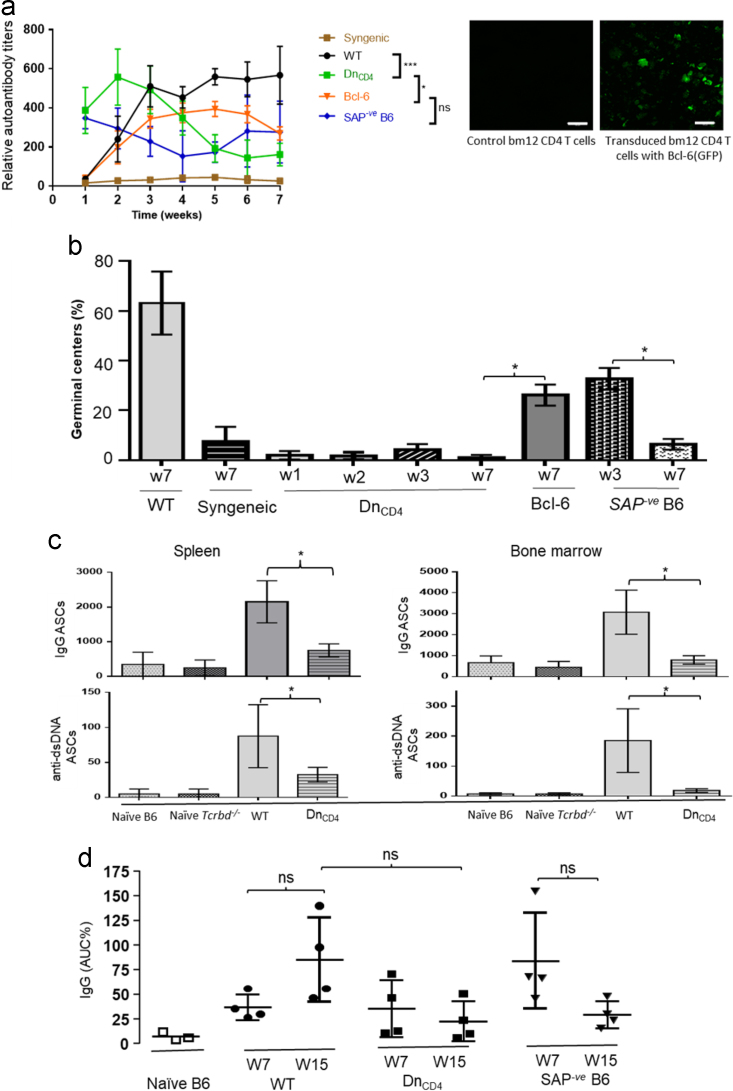
Fig. 5.
Characterisation of humoral autoimmune responses following adoptive transfer of allogeneic CD4 T cells. a) Anti-nuclear IgG autoantibody responses were examined following adoptive transfer of WT bm12 CD4 T-cells into Tcrbd−/− or SAP-ve B6 recipients, or following adoptive transfer of bm12 CD4 T cells that had been lentivirally transduced ex vivo with GFP-Bcl-6 (Bcl-6). Right panel representative imaging depicting GFP-Bcl-6 expression in transduced bm12 CD4 T-cells and control bm12 CD4 T-cells without Bcl-6-GFP expression. Cells were cultured with Bcl-6 LV vector and control vector with stimuli and checked for fluorescence at day 4. Scale bar 100 µm. b) Splenic germinal center activity in different test groups, 7 weeks after adoptive transfer of either WT or Bcl-6-transduced bm12 CD4 T cells. c) Seven weeks after adoptive transfer of donor bm12 CD4 T cells, IgG-secreting (top row) and anti-double-stranded DNA (dsDNA, bottom row) plasma cells were enumerated in spleen (left) and bone marrow (right) by standard ELISPOT assay and expressed as antibody secreting cells (ASCs)/million cells plated. Shown for comparison are responses in WT B6 recipients of WT bm12 donors (wild-type), and in naïve B6 and Tcrbd−/− B6 mice. d) Circulating IgG antibody titres were determined by ELISA in sera from various test groups at early and late time points following adoptive transfer of donor CD4 T cells. Data represent mean and SD of n = 4 mice per group. ns – not significant: *P < 0.05, **P < 0.01 and ***P < 0.001.
Fig. 6.
in vitro endothelial cell migration responses following addition of column-purified immunoglobulin from transplanted recipients. in vitro proliferation/migration of bm12 cultured endothelial cells in ‘scratch-wound’ assay following addition of serum immunoglobulin column-purified 7 weeks after bm12 heart transplantation from: WT B6 recipients (WT); Tcrbd−/− B6 recipients (DnCD4); and Sh2d1a−/− B6 recipients that are genetically deficient in SLAM-associated protein (SAP) and that do not form TFH cells (SAP-veB6). Migration was not observed with the negative fraction following column purification of sera from the WT group (histogram). Representative photomicrographs of migration into the scratch wound are included. As positive control, commercial anti-H-2Db mAb was added to endothelial cells. Data represent mean and SD of n = 4 mice per group, with discrete data-points, depicting samples from individual animals. *P < 0.05. Image scale bars are 50 µm.
2. Experimental design, materials and methods
2.1. Adoptive transfer of purified lymphocyte subsets
Column purified 2×106 CD4 T cells, obtained from either bm12 or B6 animals or both together in an 1:1 ratio, were injected intravenously (i.v) into naive Tcrbd−/− mice. Cells were purified as described previously [1], [2]. Spleens were harvested from challenged animals at day 50 after adoptive transfer for characterisation of follicular architecture and compared to control non-reconstituted naïve Tcrbd−/− animals. Tissues were embedded in OCT compound (VWR International, USA), flash frozen in liquid nitrogen, and stored at − 80 °C. Frozen tissues were cut into 7 µm serial sections and placed onto poly-L-Lysine coated slides (Sigma Aldrich Inc.). After drying for 30 min, the tissue sections were fixed in acetone for 10 min. Sections were then air dried for 30 min and stored at −80 °C. When ready for staining, spleen sections were rehydrated with 1% phosphate buffered solution (PBS; OXOID, Hampshire, UK). Sections were stained with hematoxylin (H) (Sigma Aldrich, HHS16-500 ml) and washed with deionized water and tap water to allow stain to develop. Sections were immersed into acid ethanol and then washed with deionized water and tap water. After blotting the excess water from the slides, eosin stain (Sigma Aldrich, HT110216-500 ml) was added for one minute followed by wash with 95% ethanol, 100% ethanol and then xylene. After drying, slides were fixed with coverslip slides using permount (Fisher Scientific, SP15-100). After drying, slides were examined under microscope (Olympus, Japan) for characterisation of follicular architecture. Images were photographed using an ORCA-ER digital camera (Hamamatsu Photonics, Japan) and acquired with CellR 2.6 software (Olymous Imaging Solutions, Germany).
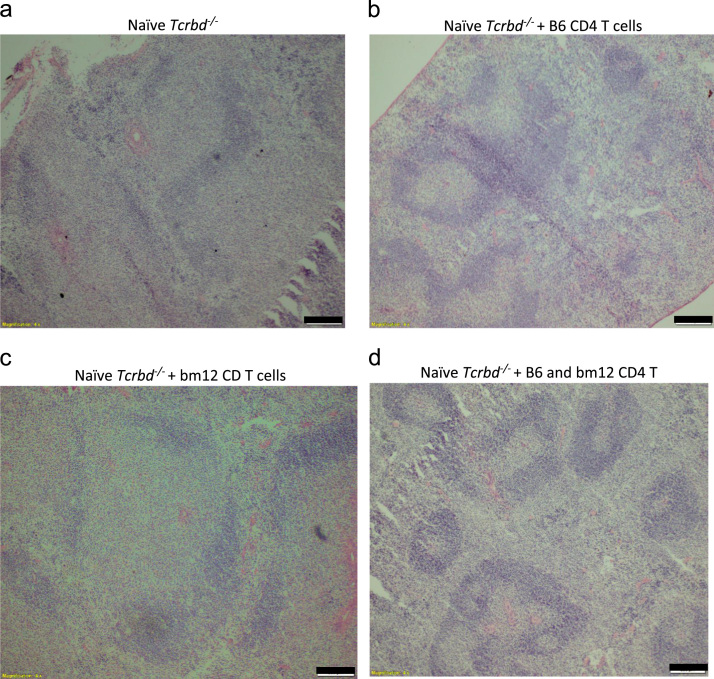
On analysis naïve Tcrbd−/−did not demonstrate any follicular architecture and mice reconstituted with either bm12 or B6 CD4 T cells developed follicular architecture, but simultaneous reconstitution of Tcrbd−/− with both bm12 and B6 CD4 T cells resulted in the development of secondary follicular architecture with classical light and dark zone orientation (Fig. 1).
Fig. 1.
Restoration of splenic B-cell follicular architecture upon reconstitution of T cell compartment in Tcrbd−/− mice. Low-power photomicrographs of H&E stained cryostat splenic sections of non-reconstituted T cell deficient Tcrbd−/− mouse (a); and naïve Tcrbd−/− mice, 50 days after reconstitution with: column purified B6 CD4 T cells (2×106 cells) (b); purified bm12 CD4 T cells (2×106 cells) (c); purified B6 CD4 T cells (2 × 106 cells) and simultaneous challenge with purified bm12 CD4 T cells (2×106 cells). Classical B cell architecture is readily evident in reconstituted mice, with characteristic light and dark zone secondary germinal center follicles observed in mice simultaneously challenged with purified bm12 CD4 T cells (d). Scale bars 200 µm.
2.2. Histopathology and immunofluorescence
Donor bm12 animals were primed with recipient B6 skin graft as described in the accompanying research article [3]. The primed bm12 allografts were then subsequently transplanted into B6 (WT) recipients. Recipient sera and spleens were examined for development of autoantibodies by HEp-2 indirect immunofluorescence as described previously [4], [5] and for development of GCs as described in the accompanying research article [3] respectively. Furthermore, allografts were monitored for rejection [3], [6]. Compared to recipients of allografts from unmodified bm12 donors, recipients of heart allografts from sensitized donors developed augmented autoantibody responses (Fig. 2a), in keeping with the presence of robust splenic germinal center activity (Fig. 2b). This was associated with accelerated rejection of heart allografts (Fig. 2c).
Recipient spleens were stained for GC markers and enumerated as described in the accompanying research article [3], and were further characterized by measuring the area occupied by GL7+ve B220+ve staining per follicle , expressed as percentage GC area per secondary follicle, using CellR 2.6 software (Olymous Imaging Solutions, Germany). Splenic GC area was significantly higher in WT (39.6 ± 14.7%) compared to Tcrbd−/− recipients (DnCD4 group) (1.0 ± 0.6 %) and WT recipients who received CD4 depleted donor bm12 allograft (RcCD4) (1.1 ± 0.51 %). However, GC area was comparable between WT and Tcrbd−/− recipients reconstituted with WT B6 CD4 T cells at the time of transplantation of bm12 allografts (53.8 ± 21.9%) (Fig. 3).
2.3. Flow cytometric identification of T follicular helper cells and GC B cells
Flow cytometric identification of TFH cells and GC B cells was performed in CD45.1 B6 recipient splenocytes following adoptive transfer of donor CD45.2 bm12 CD4 T cells or CD45.1 B6 CD4 T cells as described previously [7], [8]. Recipients were sacrificed 23 days after the adoptive transfer and splenocytes were analysed for TFH cells GC B cells. Analysis revealed a small percentage of recipient (CD45.1+ve) CD4 T cells acquiring TFH cell signature markers compared to recipient (CD45.2+ve) CD4 T cells at this time point (Fig. 4a and b). Furthermore, recipient B cells were examined for acquisition of GC markers by calculating Ki67+ve and Bcl-6+ve B cells as described previously [8]. On analysis of B cells, 3.5% of B cells were found to be positive for Ki67 and Bcl-6 characterising GC B cells on flow, a representative flow cytometry figure is shown in Fig. 4c.
2.4. Quantification of humoral autoantibody responses
Humoral reposes were examined in WT, Tcrbd−/−, SAP-ve B6 and syngeneic recipients following adoptive transfer of either unmodified (WT) bm12 CD4 T cells or following adoptive transfer of bm12 CD4 T cells that had been lentivirally transduced ex vivo with GFP-Bcl-6, as previously described [9], [10], [11]. Column purified 2 × 106 donor CD4 T cells were adoptively transferred into recipients [1]. IgG autoantibody responses were examined by HEp-2 indirect immunofluorescence (The Binding Site, Birmingham, UK) as described previously [1], [5], splenic GC activity was assessed as described in the accompanying research article [3] and total IgG levels were measured thereafter by murine pan IgG enzyme-linked immunosorbent assay (ELISA) assay as described below.
Briefly, 96-well Nunc ELISA plates (Immulon 4HBX, Thermo, Milford, MA) were coated with 1 μg/well primary goat anti-murine IgG antibody (catalogue number 1037-01; Southern Biotech, Birmingham, USA) diluted in Na2CO3-NaHCO3 buffer [pH 9.6] and incubated for overnight at 4°C. Nonspecific binding sites were blocked with phosphate-buffered saline (PBS) with 0.1% tween 20 and 5% semi skimmed milk powder for 2 hours at room temperature. After washing with PBS/0.05% tween 20 [Sigma, Poole, UK] in PBS throughout, samples were diluted 1:9 in block and added as serial tripling dilutions (50 µl/well). After incubation at 37°C for one hour, bound IgG was detected using secondary goat anti-murine IgG conjugated to HRP (catalogue number 1037-05; Southern Biotech), and developed with TMB peroxidase substrate (BD). Plates were read in the FLUOstar OPTIMA plate reader (BMG Labtech, Aylesbury, U.K.) at 450 nm. For each sample, an absorbance versus dilution curve was plotted and the area under the curve calculated as a percentage of the AUC of a standard of serial diluted murine IgG (I5381-5 mg; Sigma-Aldrich, UK) that was assigned an arbitrary value of 100% [12]. Pooled sera from naïve B6 animals were used as a negative control.
Furthermore, the development of splenic and bone marrow plasma cells with specificity for dsDNA and IgG was identified by Enzyme-linked immunospot (ELISpot) assay as described previously [1].
Whereas WT recipients developed long-lasting IgG anti-nuclear autoantibody responses, the responses in the Tcrbd−/− and SAP-ve B6 groups were truncated, and decayed after 2 weeks (Fig. 5a). Challenge of Tcrbd−/− mice with Bcl-6 transduced bm12 CD4 T cells prevented this decay and partially restored the late autoantibody response to levels observed in challenged WT B6 mice (Fig. 5a). Splenic GC activity in the challenged mice at seven weeks mirrored the autoantibody response, with GCs readily detectable in those mice with long-lived autoantibody responses. Modest GC activity was observed in Tcrbd−/− mice challenged with Bcl-6 transduced bm12 CD4 T cells (Fig. 5b). The absence of late GC activity in challenged T cell deficient B6 mice (DnCD4 group) likely explains the significantly lower number of IgG-secreting plasma cells and dsDNA-specific plasma cells in the spleen and bone marrow, when compared to challenged WT B6 recipients (WT, Fig. 5c). Similarly, measurement of total circulating IgG antibody by ELISA assay in challenged WT, Tcrbd−/−, and SAP-ve B6 recipients of bm12 CD4 T cells revealed higher levels of IgG levels in WT group , when compared to the Tcrbd−/− and SAP-ve B6 recipient groups, but this did not reach statistical significance (Fig. 5d).
2.5. in vitro endothelial cell migration assays
The response of culture bm12 endothelial cells to autoantibody was assessed by in vitro endothelial cell migration assay, using whole sera or column purified immunoglobulin isolated from recipient mice sera at different time points after transplantation. Briefly, for endothelial cell culture, 10–14 day old neonatal bm12 hearts were digested with collagenase and endothelial cells labelled with biotin-conjugated antibodies against CD31 (clone MEC 13.3, BD Pharmingen), CD105 (clone MJ7/18, BioLegend, San Diego, CA, USA) and Isolectin B4 (clone B-1205, Vector, Burlingame, CA), and then separated using anti-biotin MicroBeads (Mitenyi Biotec, Bergisch Gladbach, Germany) with an AutoMACS™ Separator (Mitenyi Biotec). Endothelial cells were cultured until 80–90% confluent and cells were subsequently incubated with medium lacking growth factors for 24 h to minimize baseline proliferation. A linear lesion was made in the cell monolayer across the diameter of the dish using a sterile pipette tip. Cells were incubated with column-purified (NAb™ Protein G Spin Purification Kit, Pierce, Rockford, IL, USA) immunoglobulin derived from sera of transplanted mice, or with sera for a further 24–36 h, fixed with paraformaldehyde (BD Cytofix kit, BD Biosciences), and then stained with 0.05% Crystal Violet solution. The negative fraction from column purification was used as negative control. For positive control, commercial anti-H-2Db mAb (BD Pharmingen, San Diego, CA, USA) was added to endothelial cells (diluted in medium in 1/200). For each plate, five fields along the lesion were analysed and numbers of cells encroaching the lesion were counted using light microscopy. The EC migration assay in response to test sera purified from transplanted recipients is detailed in the main accompanying article [3]; here we detail the EC response to addition of immunoglobulin derived from column-purified recipient sera. Significantly higher number of bm12 ECs migrated across the scratch wound line in response to column-purified sera from WT recipients of bm12 heart transplants, compared to sera from T cell deficient Tcrbd−/− (DnCD4) B6 recipients or from Sh2d1a−/− B6 recipients that are genetically deficient in SLAM-associated protein (SAP) and that do not form TFH cells (SAP-veB6, Fig. 6).
2.6. Creation of mixed haematopoietic chimeric mice
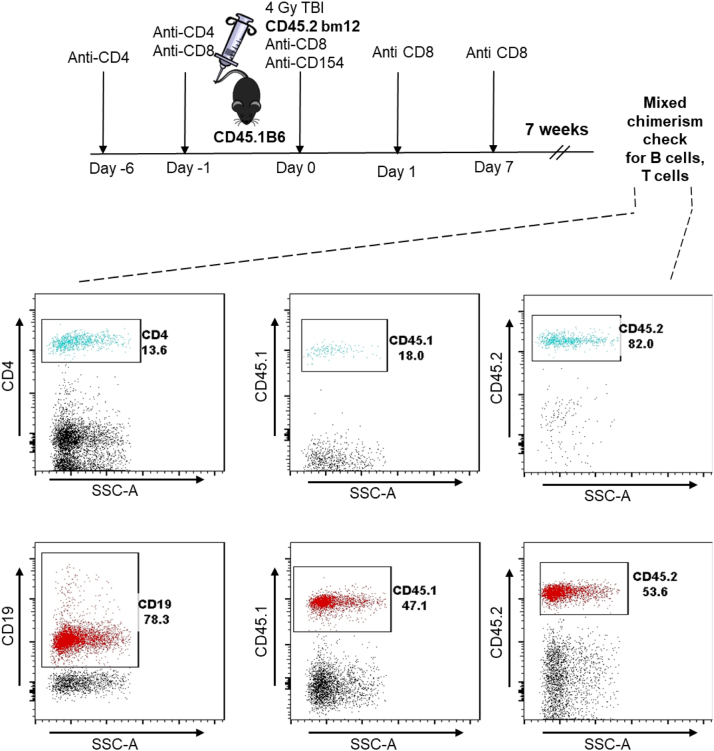
Reconstitution of Tcrbd−/− CD45.2 B6 recipients with congenic CD4 T cells was achieved by i.v injection of 1 × 107 column-purified CD4 T cells from congenic CD45.1 B6 mice (using anti-mouse CD4 MicroBeads [Mitenyi Biotec, Bergisch Gladbach, Germany] and autoMACS Separator [Mitenyi Biotec]). Mixed haematopoietic chimeric mice were created using a combination of depleting antibodies and total body irradiation (TBI), as detailed previously [13]. Briefly, CD45.1 B6 recipients were treated with anti-CD4 (YTS) on day-6 and -1 and anti-CD8 on day-1, 0, 1, and days 6–8. On day 0, recipients received 4 Gy TBI and a single dose of 2 mg of anti-CD154 mAb (MR1). Following conditioning on day 0, recipients also received an iv injection of 3 × 107 purified CD45.2 bm12 bone marrow cells. Chimerism was confirmed by flow cytometric analysis of peripheral blood lymphocytes 4 weeks after reconstitution (Fig. 7).
Fig. 7.
Creation of mixed haematopoietic chimeric mice. Stable bm12→C57BL/6 mixed haematopoietic bone marrow chimeric mice were created by conditioning sub-lethally irradiated (4 Gy total body irradiation) congenic CD45.1 B6 mice with anti-CD8, anti-CD4, and anti-CD154 mAbs, followed by intravenous injection with CD45.2 bm12 bone marrow cells (diagram). The presence of mixed haematopoietic chimerism was assessed 7 weeks later by flow cytometric analysis of peripheral blood mononuclear cells (representative histogram plots depicted), and identifying bm12 donor (CD45.2) and B6 recipient (CD45.1) fractions within gated T cell (top row) and B cell (bottom row) populations.
2.7. Statistical analysis
Data were presented as mean ± S.D. where appropriate. Mann Whitney tests were used for analysis of non-parametric data. Two-way ANOVA was employed for comparison of antinuclear anti-vimentin autoantibody and pan IgG antibody responses. Graft survival was depicted using Kaplan-Meier analysis and groups compared by log-rank (Mantel-Cox) testing. Analyses were conducted using GraphPad 4 (Graph- Pad Software, San Diego, CA, USA). Values of P < 0.05 were considered significant.
Acknowledgements
This work was supported by a British Heart Foundation Clinical Research Training Fellowship (FS/12/87/29899) to MSQ. MC was supported by the Agency for Science Technology and Research (A*STAR), Singapore. JA was supported by a grant from King Saud University, Kingdom of Saudi Arabia. JMA was supported by a Wellcome Trust Clinical Research Training Fellowship and Raymond and Beverly Sackler Scholarship. RM was supported by a European Society of Organ Transplantation Junior Basic Science Grant. MAL is supported by the Bioscience and Biotechnology Research Council. The authors acknowledge support from the National Institute of Health Research Cambridge Biomedical Research Centre and the NIHR Blood and Transplant Research Unit in Organ Donation and Transplantation at the University of Cambridge in collaboration with Newcastle University and in partnership with NHS Blood and Transplant (NHSBT).
Author contributions
MSQ, RZM and GJP were involved in conceptualisation, methodology, project administration, management of resources, formal analysis of data and writing original draft and review. MSQ and GJP acquired funding for the project. MSQ, RMZ, JA, MC, JAM, CD, TMC, ML were involved in investigation, project administration and management of resources. MG performed formal analysis to asses allograft vasculopathy.
Footnotes
Transparency data associated with this article can be found in the online version at https://doi.org/10.1016/j.dib.2018.12.078.
Transparency document. Supplementary material
Supplementary material
References
- 1.Motallebzadeh R., Rehakova S., Conlon T.M., Win T.S., Callaghan C.J., Goddard M. Blocking lymphotoxin signaling abrogates the development of ectopic lymphoid tissue within cardiac allografts and inhibits effector antibody responses. Faseb J. 2012;26:51–62. doi: 10.1096/fj.11-186973. [DOI] [PubMed] [Google Scholar]
- 2.Harper I.G., Ali J.M., Harper S.J., Wlodek E., Alsughayyir J., Negus M.C. Augmentation of recipient adaptive alloimmunity by donor passenger lymphocytes within the transplant. Cell Rep. 2016;15:1214–1227. doi: 10.1016/j.celrep.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qureshi M.S., Alsughayyir J., Chhabra M., Ali J.M., Goddard M.J., Devine C. Germinal center humoral autoimmunity mediates progression of allograft vasculopathy independently from recipient alloimmunity. J. Autoimmun. 2018 doi: 10.1016/j.jaut.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Motallebzadeh R., Bolton E.M., Pettigrew G.J. Lymphoid tissue formation in allografts: innocent until proven guilty. Transplantation. 2008;85:309–311. doi: 10.1097/TP.0b013e318162d2d0. [DOI] [PubMed] [Google Scholar]
- 5.Ali J.M., Negus M.C., Conlon T.M., Harper I.G., Qureshi M.S., Motallebzadeh R. Diversity of the CD4 T cell alloresponse: the short and the long of it. Cell Rep. 2016;14:1232–1245. doi: 10.1016/j.celrep.2015.12.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Almond P.S., Matas A., Gillingham K., Dunn D.L., Payne W.D., Gores P. Risk factors for chronic rejection in renal allograft recipients. Transplantation. 1993;55:752–756. doi: 10.1097/00007890-199304000-00013. (discussion 6-7) [DOI] [PubMed] [Google Scholar]
- 7.Linterman M.A., Rigby R.J., Wong R.K., Yu D., Brink R., Cannons J.L. Follicular helper T cells are required for systemic autoimmunity. J. Exp. Med. 2009;206:561–576. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linterman M.A., Beaton L., Yu D., Ramiscal R.R., Srivastava M., Hogan J.J. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J. Exp. Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao J., Lever A.M. Lentivirus-mediated gene expression. Methods Mol. Biol. 2007;366:343–355. doi: 10.1007/978-1-59745-030-0_20. [DOI] [PubMed] [Google Scholar]
- 10.Zhao J., Pettigrew G.J., Bolton E.M., Murfitt C.R., Carmichael A., Bradley J.A. Lentivirus-mediated gene transfer of viral interleukin-10 delays but does not prevent cardiac allograft rejection. Gene Ther. 2005;12:1509–1516. doi: 10.1038/sj.gt.3302547. [DOI] [PubMed] [Google Scholar]
- 11.Johnston R.J., Poholek A.C., DiToro D., Yusuf I., Eto D., Barnett B. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callaghan C.J., Rouhani F.J., Negus M.C., Curry A.J., Bolton E.M., Bradley J.A. Abrogation of antibody-mediated allograft rejection by regulatory CD4 T cells with indirect allospecificity. J. Immunol. 2007;178:2221–2228. doi: 10.4049/jimmunol.178.4.2221. [DOI] [PubMed] [Google Scholar]
- 13.Nikolic B., Onoe T., Takeuchi Y., Khalpey Z., Primo V., Leykin I. Distinct requirements for achievement of allotolerance versus reversal of autoimmunity via nonmyeloablative mixed chimerism induction in NOD mice. Transplantation. 2010;89:23–32. doi: 10.1097/TP.0b013e3181c4692e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material