Abstract
The chronic reduction of arterial blood pressure by thiazide diuretics (TZD) in hypertensive patients is mediated through an extra-renal mechanism. It is widely held that this extra-renal mechanism is a direct TZD inhibition of vasoconstriction. This study tested whether the TZD, hydrochlorothiazide (HCTZ), inhibited agonist constriction of mesenteric arterioles ex vivo. Mice deficient in the kidney distal convoluted tubule Na+/Cl− cotransporter (NCC), i.e., the target of thiazide inhibition–mediated diuresis, and wild type (WT), were subjected to Na+-restricted diet. Mesenteric arterioles from NCC knockout and WT mice were then isolated, placed under constant pressure, and the inhibitory effects of HCTZ (100 μM) on phenylephrine constriction determined. HCTZ did not inhibit phenylephrine constriction of arterioles from NCC knockout and wild type (WT) mice subjected to Na+-restricted diet. This study suggests that future investigations to identify the extra-renal site of chronic TZD treatment should (1) focus on indirect inhibition of vascular constriction and (2) be determined under clinically relevant conditions. These conditions include chronic TZD at relevant concentrations in hypertensive animals.
Keywords: Na+/Cl− cotransporter (NCC), Hydrochlorothiazide (HCTZ), Mesentery, Constriction, Phenylephrine
Introduction
The known target of the commonly used antihypertensive agents, the thiazide diuretics (TZD), is the kidney distal convoluted tubule Na+/Cl− cotransporter (NCC; Hughes 2004; Duarte and Cooper-DeHoff 2010; Pourafshar et al. 2018). Inhibition of Na+/Cl− cotransporter (NCC) by TZD results in diuresis and consequent decreased plasma volume, thereby lowering arterial blood pressure (Tobian and Coffee 1964; Shah et al. 1978; Hughes 2004; Ellison and Loffing 2009; Duarte and Cooper-DeHoff 2010; Shahin and Johnson 2016; Pourafshar et al. 2018).
However, the therapeutic efficacy of chronic TZD treatment is actually independent of diuresis and decreased plasma volume (Hughes 2004; Ellison and Loffing 2009; Duarte and Cooper-DeHoff 2010; Shahin and Johnson 2016; Pourafshar et al. 2018). Further, it is widely considered that the clinically relevant lowering of arterial pressure by TZD is mediated through an extra-renal action (Hughes 2004; Duarte and Cooper-DeHoff 2010; Pourafshar et al. 2018). Although this extra-renal target remains unidentified, there is evidence that TZD directly inhibit vasoconstriction (Hughes 2004; Ellison and Loffing 2009; Duarte and Cooper-DeHoff 2010; Shahin and Johnson 2016; Pourafshar et al. 2018; Table 1).
Table 1.
Effect of thiazide diuretics on vascular constriction ex vivo
| Species | Vessel | Method | Inhibition | Reference |
|---|---|---|---|---|
| Guinea pig | Mesenteric artery | Isometric | + | Calder etal. 1992, 1993, 1994 |
| Mesenteric artery | Isometric | + | Pickkers et al. 1999 | |
| Mesenteric artery | Isometric | + | Pickkers and Hughes 1995 | |
| Human | Subcutaneous artery | Isometric | + | Calder etal. 1992 |
| Mesenteric artery | Isometric | + | Colas et al. 2001 | |
| hypertensive | Mesenteric artery | Isometric | + | Colas et al. 2001 |
| Mouse | Aorta | Isometric | − | Alshahrani etal. 2017 |
| Mesenteric arterioles | Pressurized | − | Rapoport et al. 2018 | |
| Rabbit | Aorta | Isometric | − | Daniel and Nash 1965 |
| Rat | Aorta | Isometric | +/−a | Abrahams etal. 1996, 1998 |
| Aorta | Isometric | − | Colas et al. 2000b | |
| Aorta | Isometric | + | Zhu et al. 2005 | |
| Femoral artery | Isometric | + | Sladkova et al. 2007 | |
| Mesenteric artery | Isometric | +/−a | Abrahams etal. 1996 | |
| Portal vein | Isometric | − | Abrahams etal. 1996 | |
| Pulmonary artery | Isometric | +/−a | Abrahams etal. 1996 | |
| spontaneously hypertensive | Aorta | Isometric | + | Colas et al. 2000a, b, c |
Inhibition requires plasma or albumin; +, inhibition; −, no effect
While our recent finding that HCTZ decreased arterial pressure in NCC knockout (KO) mice subjected to Na+-restricted diet supports the presence of a non-renal TZD target, HCTZ failed to inhibit agonist constriction of aorta from NCC KO mice subjected to Na+-restricted diet (Alshahrani et al. 2017; Table 1). Although these findings suggest that the extra-renal TZD target is not the vasculature, the possibility remains that, in contrast to a conduit vessel such as aorta (Alshahrani et al. 2017; Table 1), TZD inhibit constriction in a resistance type vessel.
Indeed, demonstrations of TZD inhibition of constriction of isolated vessels are limited because only non-resistance-type vessels were used (Table 1). Additionally, the methodology utilized to investigate the inhibitory effects of the TZD on constriction, i.e., isometric constriction, is less physiologic than, e.g., vessel diameter measurements in pressurized vessels (Table 1). We tested, therefore, whether HCTZ inhibited constriction to the α1 adrenergic receptor agonist, phenylephrine, in second-order branches under constant pressure, from mesenteric arterial bed of NCC KO and wild type (WT) mice subjected to Na+-restricted diet.
Methods and materials
General
Wild type (WT, n = 8) and NCC KO (n = 8) mice (C57BL6, male and female; Soleimani et al. 2012) were subjected to0.55% Na+ diet for 20.3 ± 0.6 days. Secondary branches of the mesenteric vascular bed were then excised and placed in an ex vivo perfusion apparatus at 60 mmHg for diameter measurements (Nevitt et al. 2016).
Physiologic characteristics of WT and NCC KO mice/mesenteric arteriole
Age (days): WT 168.6 ± 8.8 (8), KO 150.5 ± 3.3 (8);
Weight (g): WT 27.9 ± 1.5 (8), KO 38.4 ± 2.7 (8);
Weight gain on low Na+ diet day 14 compared to day 0 (%): WT 3.2 ± 1.5 (6), KO 7.7 ± 1.7 (6), p = .0521;
Basal diameter mesenteric arteriole (μm): WT + DMSO (vehicle) 154.7 ± 10.2 (7), WT + HCTZ (100 μM) 155.1 ± 10.1 (7), NCC KO + DMSO 136.1 ± 10.7 (7), NCC KO + HCTZ 134.3 ± 10.1 (7);
Basal width mesenteric arteriole (μm): WT 25.6 ± 1.8 (8), NCC KO 28.4 ± 1.0 (8)
Constriction
Mesenteric arterioles were initially tested for their ability to constrict and the presence of functional endothelium through respective challenge with phenylephrine followed by acetylcholine. Acetylcholine (10 μM) relaxed the phenylephrine constriction of arteriolar segments from WT and NCC KO subjected to Na+-restricted diet by 90.9 ± 3.6% (5) and 70.4 ± 12.8% (8), respectively (p = 0.21). Cumulative phenylephrine concentrations were then added to mesenteric arterioles exposed to DMSO (vehicle) and, after wash, 100 μM HCTZ added followed by phenylephrine. Amount of myogenic tone of WT and NCC KO arterioles from mice subjected to Na+-restricted diet, determined by exposure to Ca2+-free solution and supramaximal sodium nitroprusside concentration, was not significantly different (5.5% ± 0.9% (6) and 6.2% ± 1.3(7) of basal diameter, respectively).
Histology/immunohistochemistry
Mesenteric arterioles were placed in 10% formalin and histology (hematoxylin-eosin, Verhoeff-Van Gieson, and Masson’s trichrome staining) and immunohistochemistry (smooth muscle actin) were performed according to standard procedures. Evaluation included blinded observer.
Calculations and statistical analysis
Acetylcholine relaxation, myogenic tone, and phenylephrine constriction were calculated as percent relaxation of phenylephrine constriction, percent relaxation of basal tone, and percent basal tone, respectively. Differences between two means and concentration-constriction curves were determined with unpaired, two-tailed t test and two-way, repeated measures ANOVA, respectively. Statistical significance was accepted at p ≤ 0.05. Shown are mean ± SE (n), where n represents the number of mice.
Materials
HCTZ, phenylephrine, acetylcholine, and sodium nitroprus-side were from Sigma-Aldrich and 0.55% Na+ diet from PMI Nutrition International. DMSO served as vehicle for HCTZ.
Results
Constriction
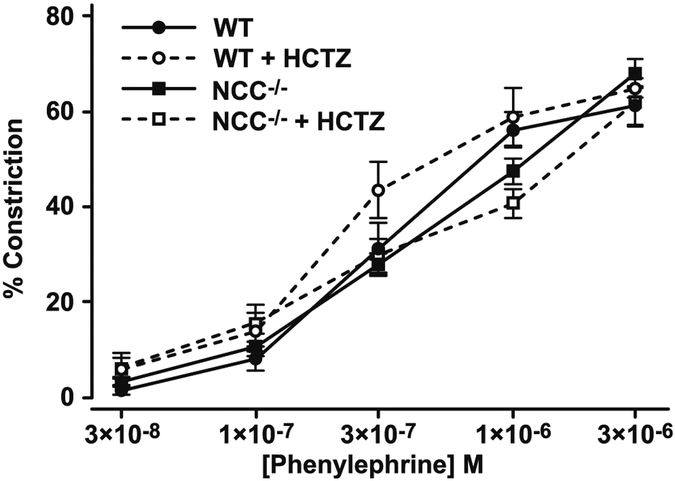
HCTZ (100 μM) did not cause a rightwards shift of the phenylephrine concentration-constriction curve of secondary branches of the mesenteric vascular bed from WT and NCC KO mice subjected to Na+-restricted diet (Fig. 1). Phenylephrine concentration-constriction curves did not differ in arteriolar segments from WT and NCC KO mice subjected to Na+-restricted diet (Fig. 1).
Fig. 1.
Effect of HCTZ on phenylephrine cumulative concentration-constriction curv6es of pressurized mouse mesenteric arterioles in vitro. WT (circles) and NCC KO mice (squares) were subjected to restricted Na+ diet. Mesenteric arterioles were then removed, pressurized, and exposed to DMSO (vehicle) followed by cumulative phenylephrine concentrations (closed symbols). Following phenylephrine washout, 100 μM HCTZ and then phenylephrine (open symbols) were added. WT: n = 6 and 3 at ≤ 1 μM and 3 μM phenylephrine, respectively; NCC KO: n = 6. The lower n with 3 μM phenylephrine was due to absence of 3 μM phenylephrine challenge, exclusion of an outlier (≈ two standard deviations from the mean) and when 3 μM phenylephrine failed or minimally constricted the unmaintained constriction to 1 μM phenylephrine
Histology/immunohistochemistry
Comparison of hematoxylin-eosin, Verhoeff-Van Gieson, and Masson’s trichrome staining, as well as immunohistochemistry of smooth muscle actin, of mesenteric arterioles from WT and NCC KO subjected to low Na+ diet did not reveal any differences (data not shown).
Discussion
The major finding of this study is that HCTZ, even at the high concentration of 100 μM, did not inhibit phenylephrine constriction in mouse pressurized arterioles from NCC KO, as well as WT mice subjected to Na+-restricted diet. These findings are consistent with the lack of effect of HCTZ inhibition of agonist constriction (isometric) of aorta from NCC KO and WT mice subjected to Na+-restricted diet (Alshahrani et al. 2017; Table 1). Also, structural changes were not observed in mesenteric arterioles, consistent with lack of structural changes in aorta from these mice (Alshahrani et al. 2017).
The inability of HCTZ to directly inhibit agonist constriction of isolated mesenteric arterioles contrasts with numerous findings of TZD, including HCTZ, inhibition of vasoconstriction of isolated vessels (Table 1). While an explanation for these contrasting findings is not entirely clear, demonstrations of TZD inhibition of constriction of isolated vessels were performed (1) in vessels derived from species other than the mouse; (2) in conduit vessels and arteries rather than resistance type vessels; and (3) under isometric conditions, i.e., conditions less physiologic than the presently used constant pressure (Table 1).
An explanation for the lack of HCTZ inhibition of constriction may have been due to possible bell-shaped, concentration-inhibition curve. This explanation is based on the use of a single, high HCTZ concentration of 100 μM. In fact, clinical HCTZ plasma concentrations were 0.26 μM (median level; Sigaroudi et al. 2018).
On the other hand, HCTZ demonstrated typical sigmoidal concentration-inhibition curves with maximal concentration of 300 μM and 1000 μM, and with approximate IC50’s of 80–100 μM (Abrahams et al. 1996, 1998; Mironneau et al. 1981; Table 1). Additionally, 100 μM HCTZ inhibited constriction of isolated vessels (Sládková et al. 2007; Table 1). Differences between preparations, including agonist potency, may also influence the findings (Dunn et al. 1994), as well as greater duration of TZD exposure.
This study suggests that future investigations to identify the extra-renal site of chronic TZD treatment should (1) focus on indirect inhibition of vascular constriction and (2) be determined under clinical conditions including chronic TZD at relevant doses in hypertensive animals.
Acknowledgements
We thank Glenn Doerman (University of Cincinnati) for the figure.
Funding sources This study was financially supported by Merit Review 5 I01 BX001000-06 award from the Department of Veterans Affairs and funds from the Center on Genetics of Transport and Epithelial Biology at the University of Cincinnati, and US Renal Care Inc. (MS), R01 AG053585 from NIA, Jewish Heritage Fund for Excellence, and the Gheens Foundation (AJL), and a Professional Development Grant from the University of Cincinnati (RMR).
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflicts of interest.
References
- Abrahams Z, Tan LL, Pang MY, Abrahams B, Tan MM, Wright JM (1996) Demonstration of an in vitro direct vascular relaxant effect of diuretics in the presence of plasma. J Hypertens 14:381–388 [DOI] [PubMed] [Google Scholar]
- Abrahams Z, Pang MY, Lam EK, Wright JM (1998) What is the plasma cofactor required by diuretics for direct vascular relaxant effect in vitro? J Hypertens 16:801–809 [DOI] [PubMed] [Google Scholar]
- Alshahrani S, Rapoport RM, Zahedi K, Jiang M, Nieman M, Barone S, Meredith AL, Lorenz JN, Rubinstein J, Soleimani M (2017) The non-diuretic hypotensive effects of thiazides are enhanced during volume depletion states. PLoS One 12:e0181376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder JA, Schachter M, Sever PS (1992) Direct vascular actions of hydrochlorothiazide and indapamide in isolated small vessels. Eur J Pharmacol 220:19–26 [DOI] [PubMed] [Google Scholar]
- Calder JA, Schachter M, Sever PS (1993) Ion channel involvement in the acute vascular effects of thiazide diuretics and related compounds. J Pharmacol Exp Ther 265:1175–1180 [PubMed] [Google Scholar]
- Calder JA, Schachter M, Sever PS (1994) Potassium channel opening properties of thiazide diuretics in isolated guinea pig resistance arteries. J Cardiovasc Pharmacol 24:158–164 [DOI] [PubMed] [Google Scholar]
- Colas B, Slama M, Collin T, Safar M, Andrejak M (2000a) Mechanisms of methyclothiazide-induced inhibition of contractile responses in rat aorta. Eur J Pharmacol 408:63–67 [DOI] [PubMed] [Google Scholar]
- Colas B, Slama M, Masson H, Colas JL, Collin T, Arnould ML, Hary L, Safar M, Andrejak M (2000b) Direct vascular actions of methyclothiazide and indapamide in aorta of spontaneously hyper-tensive rats. Fundam Clin Pharmacol 14:363–368 [DOI] [PubMed] [Google Scholar]
- Colas B, Colas JL, Masson H, Slama M, Collin T, Andrejak M (2000c) Effect of methyclothiazide on the entry of calcium into vascular smooth muscle cells. Arch Mal Coeur Vaiss 93:901–904 [PubMed] [Google Scholar]
- Colas B, Collin T, Safraou F, Chatelain D, Cordonnier C, Henry X, Safar M, Andrejak M, Slama M (2001) Direct vascular actions of methyclothiazide in remodeled mesenteric arteries from hypertensive patients. Am J Hypertens 14:989–994 [DOI] [PubMed] [Google Scholar]
- Daniel EE, Nash CW (1965) The effects of diuretic and non-diuretic benzothiadiazine and of structurally related diuretic drugs on active ion transport and contractility in smooth muscles. Arch Int Pharmacodyn Ther 158:139–154 [PubMed] [Google Scholar]
- Duarte JD, Cooper-DeHoff RM (2010) Mechanisms for blood pressure lowering and metabolic effects of thiazide and thiazide-like diuretics. Expert Rev Cardiovasc Ther 8:793–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn WR, Wellman GC, Bevan JA (1994) Enhanced resistance artery sensitivity to agonists under isobaric compared with isometric conditions. Am J Phys 266(1 Pt 2):H147–H155 [DOI] [PubMed] [Google Scholar]
- Ellison DH, Loffing J (2009) Thiazide effects and adverse effects: insights from molecular genetics. Hypertension 54:196–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AD (2004) How do thiazide and thiazide-like diuretics lower blood pressure? J Renin-Angiotensin-Aldosterone Syst 5:155–160 [DOI] [PubMed] [Google Scholar]
- Mironneau J, Savineau JP, Mironneau C (1981) Compared effects of indapamide, hydrochlorothiazide and chlorthalidone on electrical and mechanical activities in vascular smooth muscle. Eur J Pharmacol 75:109–113 [DOI] [PubMed] [Google Scholar]
- Nevitt C, McKenzie G, Christian K, Austin J, Hencke S, Hoying J, LeBlanc A (2016) Physiological levels of thrombospondin-1 decrease NO-dependent vasodilation in coronary microvessels from aged rats. Am J Physiol Heart 310:H1842–H1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickkers P, Hughes AD (1995) Relaxation and decrease in [Ca2+]i by hydrochlorothiazide in guinea-pig isolated mesenteric arteries. Br J Pharmacol 114:703–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickkers P, Garcha RS, Schachter M, Smits P, Hughes A (1999) Inhibition of carbonic anhydrase accounts for the direct vascular effects of hydrochlorothiazide. Hypertension 33:1043–1048 [DOI] [PubMed] [Google Scholar]
- Pourafshar N, Alshahrani S, Karimi A, Soleimani M (2018) Thiazide therapy in chronic kidney disease: renal and extra renal targets. Curr Drug Metab 19:1012–1020 [DOI] [PubMed] [Google Scholar]
- Rapoport RM, LeBlanc AJ, Beare JE, Soleimani M (2018) Lack of thiazide diuretic inhibition of agonist constriction of mouse mesenteric arterioles ex vivo. Naunyn Schmiedebergs Arch Pharmacol. 10.1007/s00210-018-1590-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S, Khatri I, Freis ED (1978) Mechanism of antihypertensive effect of thiazide diuretics. Am Heart J 95:611–618 [DOI] [PubMed] [Google Scholar]
- Shahin MH, Johnson JA (2016) Mechanisms and pharmacogenetic signals underlying thiazide diuretics blood pressure response. Curr Opin Pharmacol 27:31–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigaroudi A, Kinzig M, Wahl O, Stelzer C, Schroeter M, Fuhr U, Holzgrabe U, Sörgel F (2018) Quantification of hydrochlorothiazide and ramipril/ramiprilate in blood serum and cerebrospinal fluid: a pharmacokinetic assessment of central nervous system adverse effects. Pharmacology 102:133–137 [DOI] [PubMed] [Google Scholar]
- Sládková M, Kojsová S, Jendeková L, Pechánová O (2007) Chronic and acute effects of different antihypertensive drugs on femoral artery relaxation of L-NAME hypertensive rats. Physiol Res 56(Suppl 2): S85–S91 [DOI] [PubMed] [Google Scholar]
- Soleimani M, Barone S, Xu J, Shull GE, Siddiqui F, Zahedi K, Amlal H (2012) Double knockout of pendrin and Na-Cl cotransporter (NCC) causes severe salt wasting, volume depletion, and renal failure. Proc Natl Acad Sci U S A 109:13368–13373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobian L, Coffee K (1964) Effect of thiazide drugs on renovascular hypertension in contrast to their effect on essential hypertension. Proc Soc Exp Biol Med 115:196–198 [DOI] [PubMed] [Google Scholar]
- Zhu Z, Zhu S, Liu D, Cao T, Wang L, Tepel M (2005) Thiazide-like diuretics attenuate agonist-induced vasoconstriction by calcium desensitization linked to Rho kinase. Hypertension 45:233–239 [DOI] [PubMed] [Google Scholar]