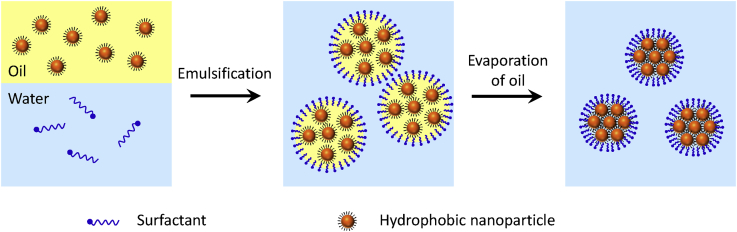
Figure 4.
Emulsion-Confined Formation of 3D Superparticles
Bai et al. demonstrated formation of BaCrO4 superparticles by using anionic surfactant sodium dodecyl sulfate (SDS) to create an oil-in-water microemulsion by ultrasonic treatment (Bai et al., 2007). Typical sizes of the assembled BaCrO4 superparticles are in the range of 100–120 nm. One important feature is that the constituent NPs retain their individual physical characteristics and do not sinter into larger particles. This is likely due to the fact that the self-assembly process relies on noncovalent interactions without chemical changes of the NPs. The surfactant bilayer surface has proved to be critical in stabilizing the superparticle. The zeta potential was measured to be a negative surface charge with potential of −27.6 mV, which is consistent with the use of SDS. In another example, when positive-charge surfactant CTAB is used to prepare Ag2Se superparticles, zeta potential characterizations indicate that these colloidal sphere assemblies have a positive charge with potential of +38.0 mV.