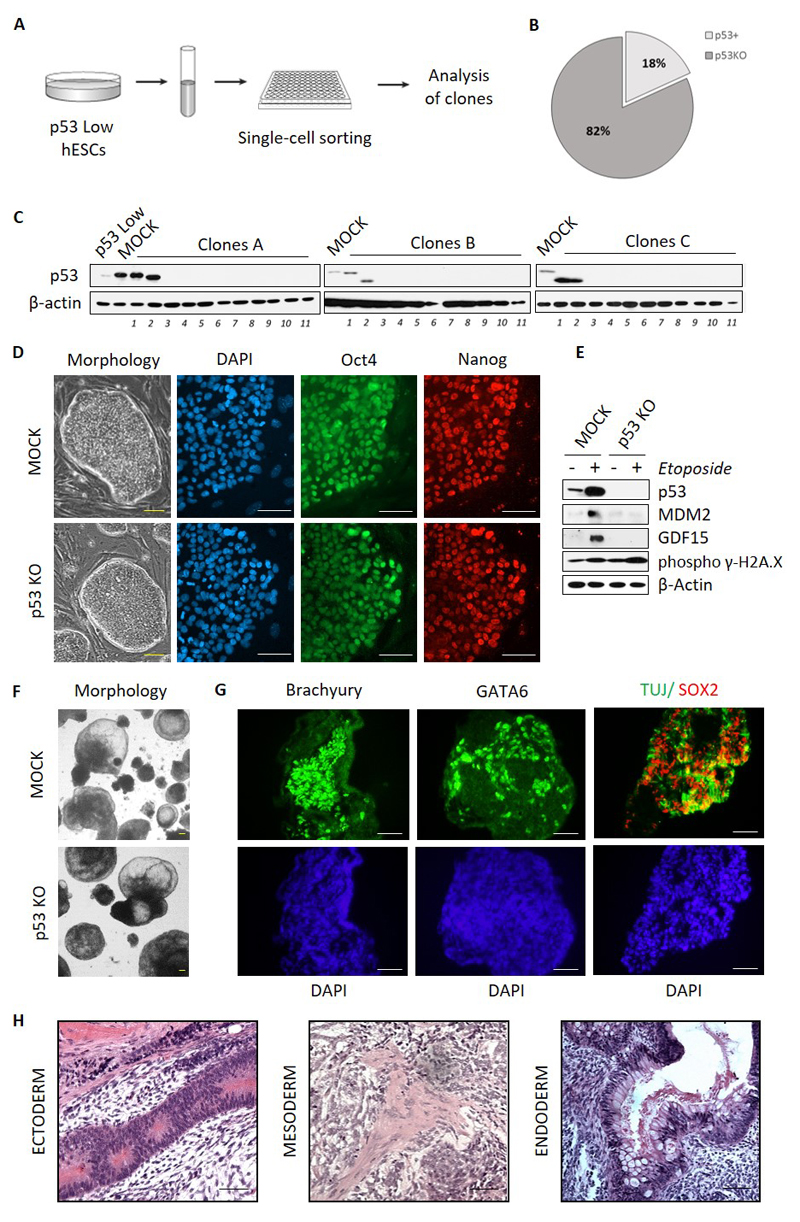
Figure 2. Efficient clonal selection of p53KO hESCs and evaluation of their stemness.
A) Experimental strategy of isolation of hESCs p53KO clones from p53 Low hESCs population. B, C) Isolated p53KO clones were analyzed by Western Blot for p53 expression, beta-actin antibody was used to verify equal loading. D) MOCK and p53 KO hESCs (clone #A11) were grown on MEFs and examined for the morphology of colonies (left) or the expression of pluripotency markers Oct4 (green) and Nanog (red) by microscopy. DAPI was used to visualize cell nuclei (blue). Scale bars = 100µm. E) MOCK and p53 KO hESCs (clone #A11) were treated either by 3.4 μM Etoposide to induce DNA damage or DMSO. After 8 hrs, cells were harvested and analyzed by Western blot for p53 protein stabilization, and the expression of p53 downstream genes – MDM2 and GDF15. DNA damage induction is shown by the increase in phospho γ-H2A.X. F) Bright field microscopy images of MOCK and p53 KO hESCs (clone#A11) following differentiation into EBs. Scale bars = 200µm. G) EBs derived from p53 KO hESCs (clone#A11) were analyzed for the expression of the differentiation markers by antibody staining and confocal microscopy. DAPI (blue) was used to visualize cell nuclei. Ectoderm markers are represented by TUJ (green) and SOX2 (red), mesoderm is represented here by Brachyury (green), and endoderm is represented here by GATA6 (green). Scale bars = 200µm. H) Hematoxylin and eosin staining of teratoma tissue from SCID mouse injected with p53KO hESCs (representative data shown for clone #A9). Tissue sections show morphological structures typical for neuroectoderm, mesoderm, and endoderm. Scale bars = 200µm.