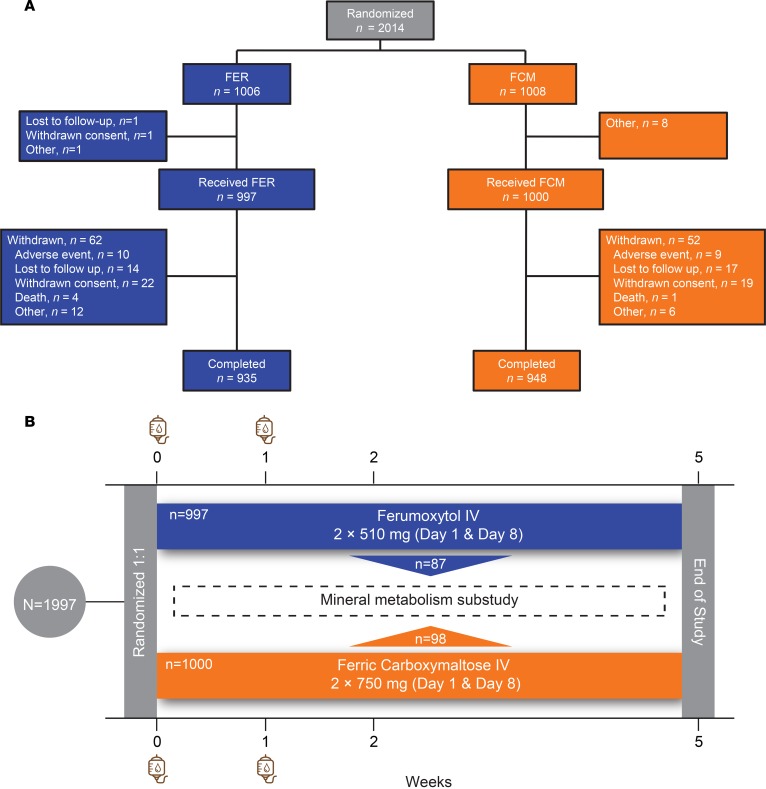
Figure 1. The FIRM trial and its nested physiological substudy.
(A) CONSORT diagram of the FIRM trial. (B) After providing written informed consent, 1,997 patients were randomized to receive intravenous (IV) ferumoxytol (n = 997) or ferric carboxymaltose (n = 1,000) on day 0 and 1 week later. Additional study visits occurred at weeks 2 and 5 after enrollment. A subgroup of US-based patients enrolled in the FIRM trial’s nested physiological substudy (ferumoxytol, n = 87; ferric carboxymaltose, n = 98) and underwent more detailed laboratory testing of phosphate homeostasis.