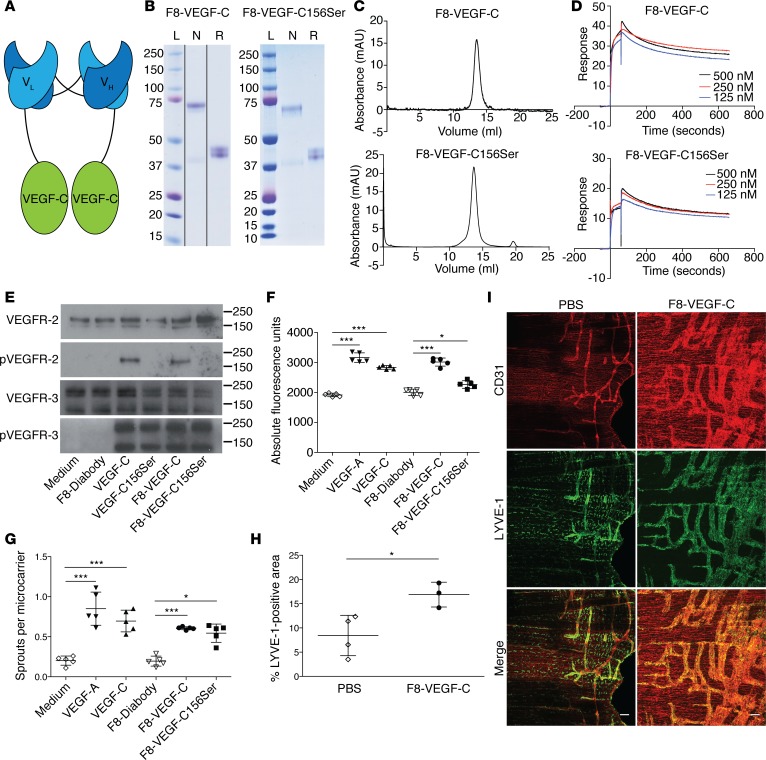
Figure 1. F8-VEGF-C and F8-VEGF-C156Ser fusion proteins retain antigen affinity and biological activity.
(A) Schematic representation of the F8-VEGF-C fusion protein. (B) SDS-PAGE analysis of F8-VEGF-C (noncontiguous lanes on same gel) and F8-VEGF-C156Ser. Lanes show size ladder (L) and protein under nonreducing (N) or reducing (R) conditions. (C) Size-exclusion chromatograms of F8-VEGF-C and F8-VEGF-C156Ser. (D) Surface plasmon resonance analysis of F8-VEGF-C and F8-VEGF-C156Ser using an EDA-coated chip. (E) Western blot analysis of VEGFR-3–overexpressing PAE cells for VEGFR-2 and phosphorylated VEGFR-2 as well as VEGFR-3 and phosphorylated VEGFR-3. Molecular weight markers are indicated on the right. (F) Proliferation assay of human LECs after 72 hours of indicated treatment (n = 5 wells per condition, 1-way ANOVA with Bonferroni post test, 1 of 3 similar experiments shown). (G) Sprouting assay of human LECs, counting sprouts formed per LEC-coated bead (n = 5 wells per condition, 1-way ANOVA with Bonferroni post test, 1 of 3 similar experiments shown). (H) Quantification of LYVE-1+ area on stained diaphragm from pups (see I, n = 3–4 animals, 2-tailed Student’s t test, 1 of 2 similar experiments shown). (I) Whole-mount immunofluorescence staining for CD31 (red) and LYVE-1 (green) on diaphragms of pups having received 5 injections of PBS or F8-VEGF-C. Scale bar: 100 μm. Data represent mean ± SD. *P < 0.05, ***P < 0.001.