Fig. 1. Interaction between MrgC11 and MOR.
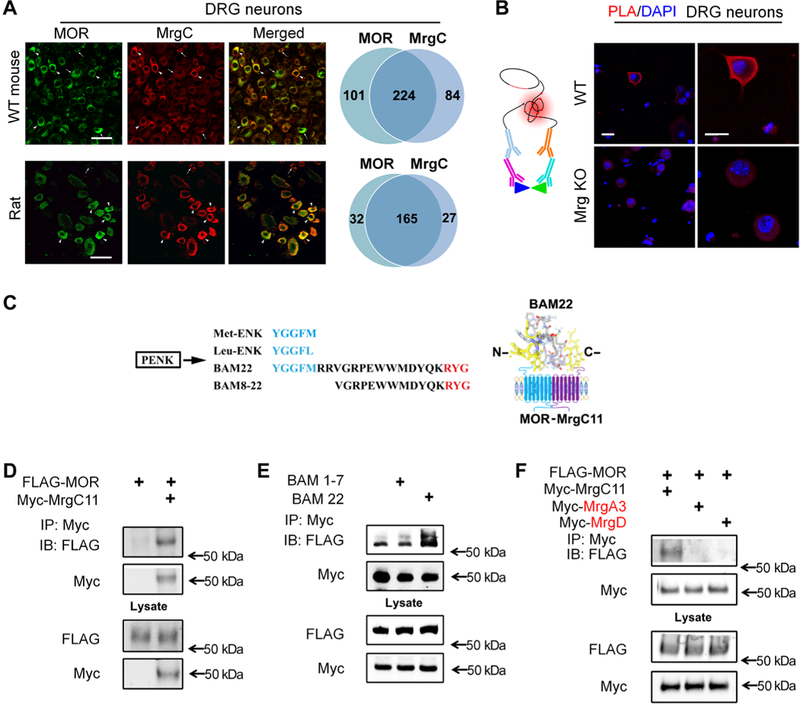
(A) Immunostaining for MrgC and MOR in DRG neurons of wild-type (WT) mice and rats (n = 3 animals per group). Arrowheads, (strongly) double-labeled cells; arrows, single-labeled cells. Right: Venn diagrams portray cells with strong coexpression. Scale bars, 50 mm. (B) Left: Schematic shows the main principles of the PLA. Right: PLA signal (red) for colocalization of MrgC and MOR antibodies in DRG neurons from WT and Mrg KO mice. DAPI (4’,6-diamidino-2-phenylindole) (blue) counterstained the nuclei. Images are representative of four experiments. Scale bars, 20 mm. (C) Left: Amino acid sequences of Met-ENK, Leu-ENK, BAM22, and BAM8–22 from the same precursor, proenkephalin (PENK), in which the consensus sequence (blue) binds the opioid receptor in the N terminus and the three-amino acid sequence (red) binds MrgC in the C terminus. Right: Diagram showing how BAM22 binds to both MOR and MrgCII. (D) Immunoprecipitation (IP) of Myc and immunoblotting (IB) of solubilized protein extracts derived from HEK293T cells transfected with Myc-MrgC11 and FLAG-MOR. (E) As described in (D), cells cotransfected with Myc-MrgC11 and FLAG-MOR were treated with bath application of full-length BAM22 or BAM1–7. (F) As described in (D), from cells cotransfected with FLAG-MOR and Myc-MrgC11, Myc-MrgA3, or Myc-MrgD. (D to F) Data are representative of three to four experiments.
