Abstract
Tissue adhesives play an important role in surgery to close wounds, seal tissues, and stop bleeding, but existing adhesives are costly, cytotoxic, or bond weakly to tissue. Inspired by the water-resistant adhesion of plant-derived tannins, we herein report a new family of bioadhesives derived from a facile, one-step Michael addition of tannic acid and gelatin under oxidizing conditions and crosslinked by silver nitrate. The oxidized polyphenol groups of tannic acid enable wet tissue adhesion through catecholamine-like chemistry, while both tannic acid and silver nanoparticles reduced from silver nitrate provide antimicrobial sources inherent within the polymeric network. These tannin-inspired gelatin bioadhesives are low-cost and readily scalable and eliminate the concerns of potential neurological effect brought by mussel-inspired strategy due to the inclusion of dopamine; variations in gelatin source (fish, bovine, or porcine) and monomer feeding ratios resulted in tunable gelation times (36 s to 8 min), controllable degradation (up to 100% degradation within a month), considerable wet tissue adhesion strengths (up to 3.7 times to that of fibrin glue), excellent cytocompatibility, as well as antibacterial and antifungal properties. The innate properties of tannic acid as a natural phenolic crosslinker, molecular glue, and antimicrobial agent warrant a unique and significant approach to bioadhesive design.
Keywords: tannin, polyphenol, gelatin, bioadhesives, antimicrobial, medical device
Graphical abstract
1. Introduction
Tissue adhesives have revolutionized surgical procedures, assuming multiple roles as wound closure, tissue sealants, and hemostatic agents to reduce blood loss and promote healing [1–7]. However, existing bioadhesives demonstrate weak adhesion strength to wet tissue, require harsh chemical reactions, or possess poor biocompatibility, thereby greatly limiting clinical applications [1–8]. For example, tissue adhesives based on cyanoacrylate (Super Glue) possess high mechanical strength but are cytotoxic and the curing process is exothermic [9], while adhesives based on poly(ethylene glycol) (PEG) swell too much and fragile in nature. Most commercially available polyurethane-based bioadhesives, such as TissuGlu, suffer from exothermic and harsh chemical reactions during adhesive curing [9, 10]. Moreover, surgical site infections are major concerns that can prolong wound healing or cause abscess formation, especially for the cases of wound care in infection prone areas such as diabetic foot ulcers, yet traditional bioadhesives lack innate antimicrobial properties.
Mussel-inspired bioadhesives have gained wide attention, mimicking the strong underwater adhesion of the blue mussel Mytilus edulis by employing catechol group containing compounds such as L-3,4-dihydroxyphenylalanine (L-DOPA) and dopamine. Our recent works have highlighted the major functionalities of catechol side groups in forming polymeric networks and tissue chemical bonding, revealing strategies for facile synthesis of catechol-functionalized biodegradable polymers with greatly improved wet adhesion strengths [11–15]. However, the prohibitive costs of such compounds [4–8] and neurological effects of dopamine pose concerns on the commercialization of such catechol-functionalized tissue adhesives [16].
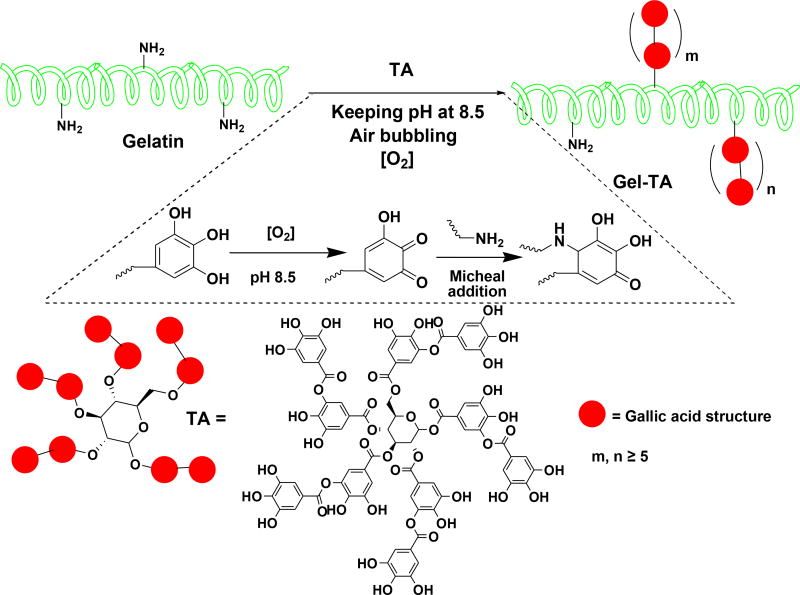
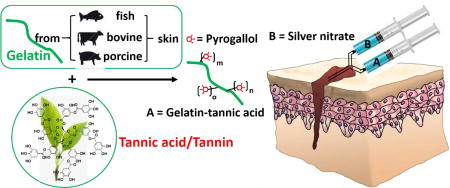
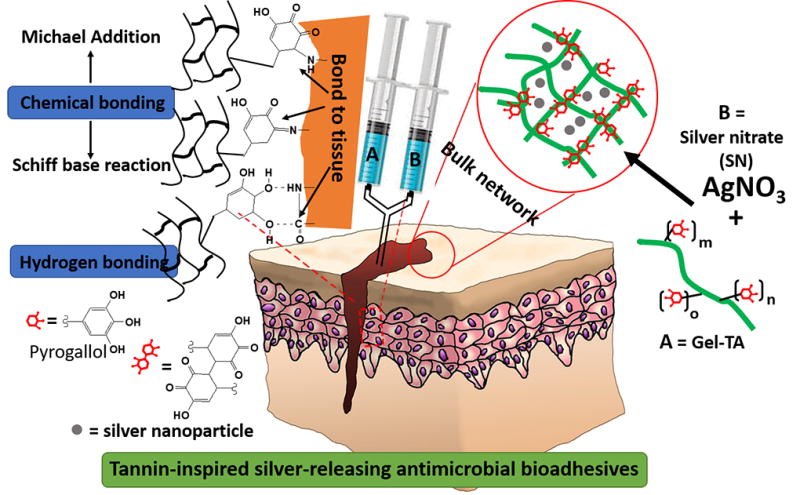
To overcome such challenges in bioadhesive engineering, plant-derived polyphenol compounds such as tannins may serve as a remarkable source of catechol/pyrogallol groups that is both safe and low-cost [17–19]. Tannic acid, in particular, meets the following criteria as a keystone ingredient in bioadhesive synthesis: (i) diverse bonding functionalities owed to its five-arm polyphenol structure, enabling dense polymeric crosslinks through hydrogen and ionic bonding or hydrophobic interactions [19, 20], (ii) covalent interactions with amino groups of peptides, leading to improved mechanical properties in gelatin-based adhesives [19, 21], (iii) pyrogallol moieties which, when oxidized, can chemically bond with tissue to yield strong tissue adhesion [21], and (iv) innate antimicrobial capabilities owed to the astringency and membrane action of oxidized tannins [22–25]. Despite these advantages, tannic acid is rarely incorporated into bioadhesive formulations, as its multiple noncovalent interactions tend to drive hydrogels toward coacervation rather than network formation [21, 26–29]. Thus, by taking advantage of the above unique combinatory features, we herein designed a new family of tannin-inspired bioadhesives produced by a facile, one-step Michael addition reaction of tannic acid and gelatin under oxidizing conditions (Scheme 1). Various sources of gelatin (fish, bovine, or porcine), tannic acid feeding ratios, and crosslink initiator content (% silver nitrate) were formulated systematically to produce a family of bioadhesives with fine tunable gelation times and degradation rates (Scheme 2). Overall, the tannin-inspired gelatin bioadhesives exhibited considerable wet tissue adhesion strength and intrinsic antibacterial and antifungal properties, promising a versatile platform for the design of surgical tissue adhesives.
Scheme 1.
Scheme 2.
2. Experimental Section
2.1 Materials
Gelatin from cold water fish skin (FGel, G7041), gelatin from bovine skin (BGel, gel strength ~ 225 g Bloom, Type B, G9382), and tannic acid (TA, 403040) were all purchased from Sigma-Aldrich and used without further purification.
2.2 General Measurements
1H-NMR spectra of modified and unmodified gelatin polymers were recorded on a 300 MHz Bruker DPX-300 FT-NMR spectrometer in DMSO-d6. Attenuated total reflection-Fourier transform infrared (ATR-FTIR) spectra were measured with a Nicolet 6700 FTIR spectrometer using polymer powder directly, with air used as background.
2.3 Synthesis of Tannic Acid Modified Gelatin (Gel-TA)
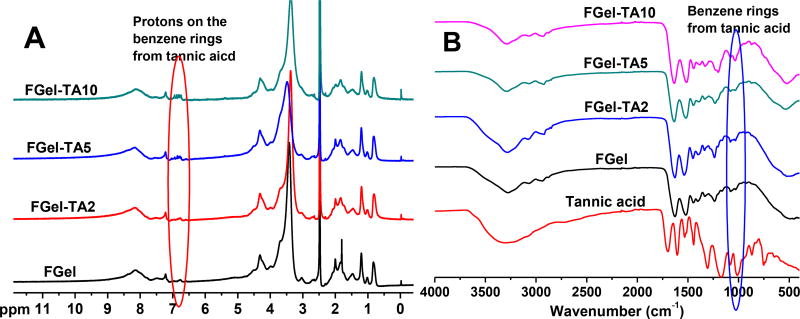
Gelatin was modified by tannic acid (TA) through the Michael Addition reaction between the amine groups of gelatin and the double bonds of catechol groups on oxidized TA, under basic condition. Briefly, gelatin (20 g) was stirred in DI water (200 mL) at 60°C for 2 hours to form a gelatin solution with a final concentration of 10 w/v%. The pH value of the solution was adjusted to 8.5 using 12M NaOH. A solution of TA was then slowly added to the gelatin solution, while stirring, at defined TA/gelatin ratios (1, 2, 5, or 10%, w/w to gelatin), and the mixture was allowed to react for another 3 hours at 60°C. The pH value of the mixture during reaction was monitored with a pH meter to maintain a constant pH of 8.5 by dropwise addition of NaOH (1 M). Air was bubbled into the systems throughout the reaction. Following the reaction, the pH value of the solution was adjusted into 7.4, and the solution was dialyzed against DI water at room temperature (for TA modified FGel, or FGel-TA) or 37°C (for TA modified BGel, or BGel-TA) using a dialysis tube with a molecular weight cut-off (MWCO) of 1000 Da. The dialyzed solution was then freeze-dried to obtain a TA modified gelatin with × wt% of TA (FGel-TAx or Fx; BGel-Tax or Bx). Gel-TAs with different gelatin sources and TA feeding ratios were synthesized, the names are listed in Table 1. 1H NMR (300 MHz; DMSO-d6; δ, ppm) of FGel-TA: 0.86–5 (m, amino acids on gelatin), 6.78–6.9 (m, protons on the benzene rings of TA). FTIR of FGel-TA (cm−1): 1638 (strong, -COO- and –CONH-), 1036 (weak, benzene rings from TA).
Table 1.
Nomenclature and feeding ratio of TA modified gelatin (Gel-TA).
| Polymer name | Gelatin used | TA feeding ratio to Gel (wt%) |
|---|---|---|
| FGel-TA1 (F1) | Gelatin from cold fish skin (FGel) | 1 |
| FGel-TA2 (F2) | FGel | 2 |
| FGel-TA5 (F5) | FGel | 5 |
| FGel-TA10 (F10) | FGel | 10 |
| BGel-TA2 (B2) | Gelatin from bovine skin (BGel) | 2 |
| BGel-TA10 (B10) | BGel | 10 |
| PGel-TA2 (P2) | Gelatin from porcine skin (PGel) | 2 |
2.4 Assessment of gelation times
2.4.1 Cross-linking of Gel-TA and measurement of set time
Gel-TA polymers, at 15 wt% in Tris-HNO3 buffer (pH 8.5), were crosslinked using equal volume of silver nitrate (SN) solutions at either room (25°C) or body (37°C) temperature (Scheme 2). The gel times (or set times) of various formulations were determined by the tilt test, repeated in triplicates and averaged (Table 2). The names of SN crosslinked Gel-TA hydrogels are also listed in Table 2.
Table 2.
Gel times of tannic acid modified gelatins at different pH values, with different tannic acid feeding ratios (1, 2, 5, or 10 wt%) or obtained from different sources (cold fish skin (F), bovine skin (B), or porcine skin (P)), crosslinked by AgNO3 solutions at different temperatures.
| Polymera | Polymer concentrationa (wt%) |
AgNO3 concentrationb (g/mL) |
pH valuec |
Test temperature (°C) |
Measured gel time (s) |
Names of crosslinked hydrogels |
|---|---|---|---|---|---|---|
| FGel-TA1 | 15 | 0.1 | ~7.4d | RTe | Uncross-linkable | - |
| FGel-TA2 | 15 | 0.1 | ~7.4d | RTe | 235 ± 35 | F2-S100 (F-100) |
| FGel-TA2 | 15 | 0.075 | ~7.4d | RTe | 282 ± 23 | F2-S75 (F-75) |
| FGel-TA2 | 15 | 0.05 | ~7.4d | RTe | 480 ± 23 | F2-S50 |
| FGel-TA2 | 15 | 0.05 | 8.5 | RTe | 229 ± 5 | - |
| FGel-TA2 | 15 | 0.05 | ~7.4d | 37 | 246 ± 8 | - |
| FGel-TA5 | 15 | 0.05 | ~7.4d | RTe | 189 ± 42 | F5-S50 |
| FGel-TA10 | 15 | 0.05 | ~7.4d | RTe | 36 ± 11 | F10-S50 |
| FGel-TA2 | 5 | 0.1 | ~7.4d | RTe | Uncross-linkable | - |
| BGel-TA2 | 15 | 0.075 | ~7.4d | RTe | 410 ± 88 | B2-S75 (B-75) |
| PGel-TA2 | 15 | 0.075 | ~7.4d | RTe | 25 ± 7 | P2-S75 |
Polymers were dissolved in Tris-HNO3 (pH 8.5) buffer solution;
Polymer solution/AgNO3 solution ratio was kept at 1/1 g/mL;
This is the pH value of polymer solution;
The pH values of Gel-TAx in Tris-HNO3 (pH 8.5) buffer solution were all around 7.4 for different polymers;
RT: room temperature.
2.4.2 Rheological evaluations
Rheological evaluations were conducted using Discovery Series Hybrid Rheometer (DHR-1, TA Instruments, USA) in a parallel plate configuration, employing sandblasted stainless steel 40 mm diameter plates throughout and a Peltier plate for temperature control. In a representative rheological test for gelling kinetics, 2 mL Gel-TA in Tris-HNO3 (15 wt%, pH 8.5) was mixed with 2 mL of silver nitrate (SN) solution in DI water (0.075 g/mL or 0.1 g/mL). This mixture was applied to the lower plate of the rheometer, preheated to a preset temperature. The upper plate was immediately brought down to a gap distance of 40 µm to begin measurement. A low frequency of 1 Hz and 1% strain was applied to minimize interference with the gelation process and to keep the measurement within the linear viscoelastic region. The gelation kinetics was measured in a time sweep by monitoring the change of storage (G′) and loss (G″) moduli as a function of time. All measurements were repeated in triplicates.
2.5 Properties of the Crosslinked Gel-TA Family
Mechanical properties of dried Gel-TAs crosslinked by SN, including tensile strength, Young’s modulus and elongation at break, were measured according to ASTM D412A on an Instron 5966 machine fitted with a 1 KN (for dry samples) or 10 N (for wet samples) load cell (Instron, Norwood, MA). Briefly, strip shaped samples (25 mm × 6 mm × 1.5 mm, length × width × thickness) were pulled at a rate of 500 mm/min and elongated to failure. The Young’s modulus was obtained by calculating the gradient from 0 to 1% (for dry samples) or 10% (for wet samples) of elongation of the stress-strain curve. Eight specimens per sample were tested and averaged. In order to evaluate the effect of hydration on the mechanical properties, the mechanical tests were also conducted on wet samples with a water content of around 10 wt% or 50 wt%.
The sol/gel content, an indication of non-crosslinked/crosslinked fractions of the hydrogel, and swelling ratio were measured by the mass differential before and after incubation of the crosslinked polymer in 1, 4-dioxane (sol content) or water (swelling ratio) as described previously [4–7]. The sol content and swelling ratio were then calculated using equations (1) and (2), respectively.
| Equation (1) |
| Equation (2) |
Here Wi represents the initial dry weight of crosslinked Gel-TA hydrogel disk, Wd represents the weight of freeze-dried sample after the un-crosslinked part was washed by 1, 4-dioxane for 48 hours, and Ws represents the network weight after the leached and dried sample was suspended in water for 24 hours. Six samples were tested for each study group (n = 6).
Degradation studies were conducted in PBS (pH 7.4) and at 37°C using cylindrical disc specimens (7 mm in diameter, 2 mm thick) as described previously [4–7]. The mass loss was calculated by comparing the initial mass (W0) with the mass measured at the pre-determined time points (Wt) using equation (3). Six samples were tested for each study group (n = 6).
| Equation (3) |
2.6 Adhesion strength of SN crosslinked Gel-TAs
The adhesion strength of Gel-TAs crosslinked by SN was determined by the lap shear strength test adapted from ASTM F2255-05 standard and method used in previous literatures [30, 31]. The detailed testing process was described in our previous study [4, 6].
2.7 Cytocompatibility Tests of Gel-TAs and Crosslinked Gel-TA Hydrogels
The cytocompatibility of Gel-TA polymers and crosslinked Gel-TA hydrogels were evaluated as described previously [6]. Briefly, human-derived mesenchymal stem cells (hMSCs, ATCC® PCS-500-012™) were purchased from ATCC and cultured in growth media (Dulbecco’s modified eagle’s medium (DMEM), 10% (v/v) fetal bovine serum (FBS) and 1% (v/v) antibiotic antimycotic solution (100×)) up to passages 5–10 prior to the cell cytotoxicity and proliferation studies. In vitro pre-polymer cytotoxicity was assessed by the MTT (methylthiazolyl-diphenyl-tetrazolium bromide) assay against hMSCs, with commercially available poly (ethylene glycol) diacrylate (PEGDA, Mn = 700Da) and unmodified gelatins (from cold fish skin or bovine skin) serving as positive and negative controls respectively. Various Gel-TA polymer formulations, gelatin, and PEGDA were prepared at concentrations of 10, 1, and 0.1 mg/mL in growth media, with pH adjusted to 7.4. To each well of a 96-well cell culture plate, 200 µL of hMSCs in growth media, at a density of 5×104 cells/mL, were added and incubated for 24 hours at 37°C, 5% CO2 and 95% relative humidity. The medium was then completely replaced by 200 µL of the above polymer solutions (10, 1, and 0.1 mg/mL in growth media), and incubated for another 24 hours prior to performing MTT assays. Viabilities of cells in Gel-TA polymer, gelatin, or PEGDA containing growth media were normalized to that of cells cultured in blank growth media.
The cytotoxicity of sol contents (or leachable fractions) and degradation products of SN crosslinked Gel-TA hydrogels were also studied using the MTT assay against hMSCs, while FDA approved poly (lactic-co-glycolic acid) (PLGA, LA/GA=50/50, Mw~60KDa, purchased from Polyscitech) served as control. The sol content solutions of Gel-TA hydrogels were obtained by incubating equal mass (0.5 g) hydrogel samples in 5 mL of PBS (pH 7.4) at 37°C for 24 hours. Next, three different dilutions were prepared for testing: 1×, 10× and 100×, where 1× was the solution of leached products with no dilution, while 10× and 100× were solutions with 10 times and 100 times dilution of the 1× solution in PBS, respectively. To each well of a 96-well cell culture plate, 200 µL of hMSCs in growth media at a density of 5×104 cells/mL were added and incubated for 24 hours. Next, 20 µL of the above sol content solutions were added and the cells were incubated for another 24 hours prior to performing the MTT assays. Six samples were tested for each study group (n = 6).
Following, cytotoxicity of the hydrogels’ degradation products were evaluated by fully degrading equal weight (1 g) of Gel-TA hydrogel samples as well as PLGA in 10 mL of 0.2 M NaOH solution. After adjusting pH to 7.4, the resultant solutions were diluted to three concentrations (1×, 10× and 100× as above) using PBS (pH 7.4), and used for MTT analysis as described above. Six samples were tested for each study group (n = 6).
All of the above solutions were pH-neutralized and passed through a sterilized 0.2 µm filter prior to use for cell culture. The cell viability results were normalized to the viability of cells in blank growth media.
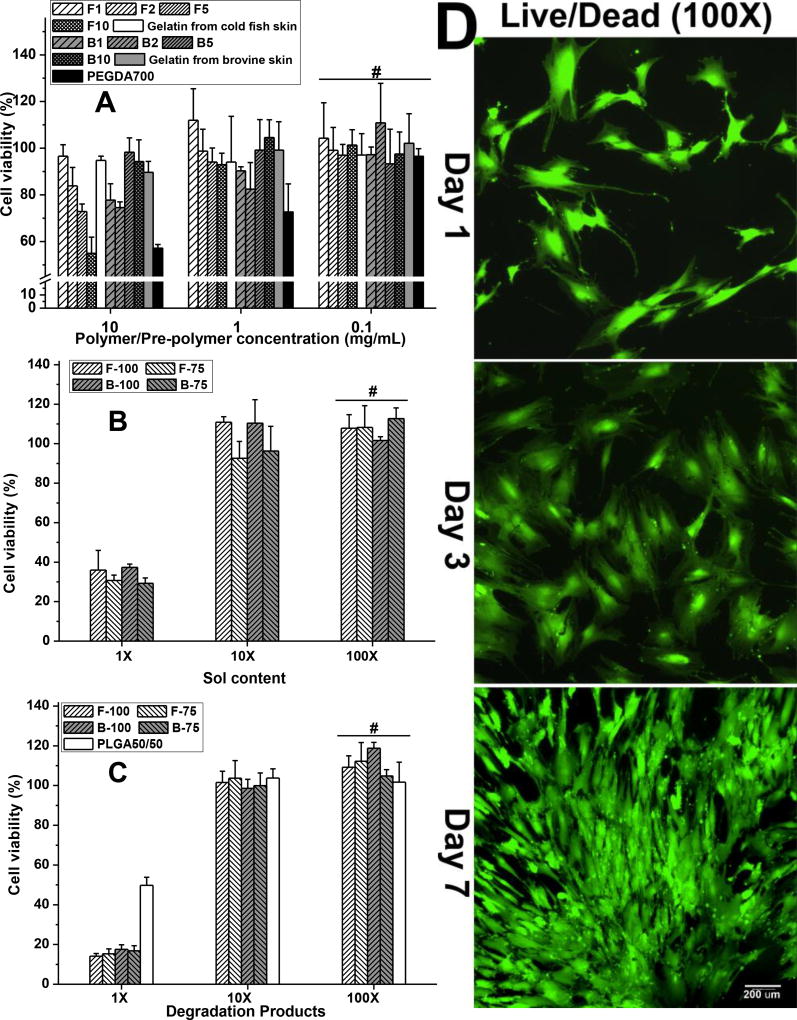
As a further assessment of film cytotoxicity, cell adhesion and proliferation of hMSCs were studied on BGel-TA2 SN 0.075g/mL (B2-S75, or B-75) as a representative film using Live/Dead staining. Briefly, 20 µL of BGel-TA2 (B2) and 20 µL of silver nitrate solution (0.075 g/mL) were mixed together and uniformly coated onto the surface of a glass slide, forming roughly 15 mm diameter thin films. The crosslinked B75 films were then sterilized by incubation in 70% ethanol for 24 hours followed by exposure to UV light for 3 hours. The samples were then placed in 24-well plates and seeded with 500 µL hMSC solutions at 5,000 cells/cm2, followed by growth media replacement the next day. At each time point (1, 3 and 7 days post cell seeding), the constructs were removed from the well plate, rinsed by PBS, and stained by Live/Dead Viability/Cytotoxicity Kit (Invitrogen, molecular probes, Eugene, OR) to observe cell morphology using an inverted light microscope (Nikon Eclipse Ti-U) equipped with a ANDOR DL-604M-#VP camera and Prior Lumen 200.
2.8 Antimicrobial Performance of Crosslinked Gel-TA Hydrogels
The anti-bacterial and anti-fungal potencies of SN crosslinked Gel-TA hydrogels were evaluated using Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) as positive and negative bacteria models, and Candida albicans (C. albicans) as a fungi model.
2.8.1 Anti-bacterial performance of SN crosslinked Gel-TA hydrogels
2.8.1.1 Bacterial incubation
Staphylococcus aureus (S. aureus, ATCC® 6538™) and Escherichia coli (E. coli, ATCC® 25922™) were purchased from ATCC (American Type Culture Collection) and used following established safety protocols. Tryptic soy broth (Cat. #: C7141) and tryptic soy agar (Cat. #: C7121) used for S. aureus culture were purchased from Criterion (via VWR). Luria Broth Base (LB broth, Cat. #: 12795-027) and Select Agar (Cat. #: 30391-023) used for E. coli culture were purchased from Invitrogen. S. aureus and E. coli were cultured at 37°C in sterilized tryptic soy broth and LB broth respectively under 150 rpm in a rotary shaker overnight, and the obtained bacteria suspensions were diluted into desired concentrations before use.
2.8.1.2 In-vitro evaluation of bacterial inhibition
The bacterial inhibition ratios of SN crosslinked Gel-TA hydrogels against S. aureus and E. coli were evaluated using F2-S75 (F-75), F2-S100 (F-100), B2-S75 (B-75), and B-2-S100 (B-100) as the representative experimental samples and PEGDA/HEMA (w/w = 1/1) as negative control [5, 6, 32]. Briefly, 0.2 g of freeze-dried hydrogels were immersed in 20 mL of germ containing broth with bacterial concentrations of around 1×106 CFUs/mL (CFU: colony forming unit) [32]. The samples were then incubated at 37°C with oscillation at a frequency of 150 rpm. Broth containing only bacteria served as another negative control. After 24 hours incubation, the bacterial inhibition ratios were determined. To eliminate the effect of silver chloride (formed by the reaction between residual SN in crosslinked Gel-TA hydrogels and NaCl in broth) dispersion on optical density (OD) of bacteria suspension, instead of OD value test, an agar-casting and colony-counting method was used to determine the bacterial inhibition ratio. Briefly, the broth of each sample was removed after 24 hours’ incubation, diluted and casted onto an agar plate for colony counting after another 24 hours incubation. For each hydrogel, at least 3 specimens were used and 2 agar-casting and colony-counting were conducted for each specimen. The bacterial inhibition ratios of hydrogels were calculated by equation (4):
| Equation (4) |
where N0 is the seeding bacteria concentration (CFUs/mL) in broth medium, Nt and Ncon are bacteria concentrations following 24 hour incubation of the hydrogel samples and pure broth control respectively.
2.8.1.3 Antibacterial evaluation by zone of inhibition
F-75, F-100, B-75 and B-100, as well as PEGDA/HEMA hydrogel (as control), were used to test anti-bacterial inhibition halos by a modified Kirby Bauer technique [6, 33, 34]. Briefly, 10 mL of S. aureus or E. coli in broth (at OD600nm values around 0.07) were respectively dispersed onto a tryptic soy or LB agar plate (Φ100×7mm). Next, hydrogel disks (Φ5mm) were placed onto the agar plate and incubated for 24 hours at 37°C. After incubation, the bacterial inhibition halos around the hydrogel samples were observed and their diameters were measured.
2.8.2 Anti-fungal performance of SN crosslinked Gel-TA hydrogels
2.8.2.1 Fungi incubation
Fungi (Candida albicans, C. albicans) was purchased from ATCC (ATCC® 10231™), and used following established safety protocols. YM medium broth (Lot #: 1964C030) and YM agar (Lot #: 1964C030) used for fungi (C. albicans) culture were respectively obtained from Amresco and Acumedia. Tween 20, for stabilizing fungi suspensions, was added to YM broth medium to obtain a final concentration of 0.5 wt% and then sterilized. Throughout the experiments, C. albicans was maintained on YM agar plates. To obtain a working concentration of fungi, C. albicans was scraped from YM agar plates, dispersed in Tween 20 containing YM broth medium, counted with a hemocytometer, and then diluted into a final fungi concentration of 0.5–1×107 cells/mL [6, 35]. Quantitative evaluation of fungal survival was obtained using a colony counting method, described below in 2.8.2.2.
2.8.2.2 Anti-fungal effect of direct exposure to hydrogels
The anti-fungal effect of direct exposure to SN crosslinked Gel-TA hydrogels was tested using F-75, F-100, B-75, and B-100 as the representative experimental groups and PEGDA/HEMA as control. Briefly, 20 mg freeze-dried hydrogel disks were placed in the wells of a 24-well tissue culture plate, and 2 mL of C. albicans suspension in Tween-20 containing YM broth medium (0.5–1×107 cells/mL) was added to each well. Samples without hydrogel were used as blank control. The 24-well plates were incubated for 3 hours at 37°C with a shaking speed of 100 rpm. Then the fungi containing medium was diluted 300 times, and 0.3 mL diluted medium was removed and cast on YM agar plates (Φ6×2 mm). After incubation at 37°C for 24 hours, fungi colonies on the YM agar plates were counted, and the fungi survival ratios were calculated according to equation (5). For each sample, at least 6 plates were cast (n = 6), and the numbers were averaged.
| Equation (5) |
Here, Ns is the number of fungal colonies for sample, and Ncon stands for the number of fungal colonies for YM broth blank control.
2.8.2.3 Halo test
The anti-fungal performance of SN crosslinked Gel-TA was also evaluated using the halo test method with PEGDA/HEMA as control. Briefly, 4 mL of YM broth medium containing 0.5–1×107 cells/mL C. albicans was evenly cast onto YM agar plates (Φ100×7 mm). The hydrogel discs (around Φ5mm) were placed on the agar plate and the constructs were incubated at 37°C for 24 hours under dark before being examined for a “halo” or “zone of inhibition” surrounding the gel disc.
2.9 Statistical Analysis
All statistical data were expressed as mean ± standard deviation. Statistical analysis was performed using Student’s t-test. Data were considered to be significant, when p < 0.05.
3. Results and Discussion
We chose animal-derived gelatins as our starting material due to their wide availability, favorable biocompatibility, and high wet mechanical strengths [6]. Typically, the modification of gelatin for adhesives employs 1-Ethyl-3-(3-dimethylamino -propyl)carbodiimide (EDC) coupling or vinyl groups (e.g. methacrylated gelatins), but former method can self-crosslink gelatin [36–39], while the latter requires a large excess of methacrylic anhydride and subsequent dialysis [40]. Here, we demonstrate that gelatin modification can be performed with tannic acid (TA) through a one-step Michael addition reaction that is low-cost, convenient, and scalable. Additionally, tannic acid is a widely used food additive that is categorized as GRAS (generally recognized as safe) [29]. Since tannic acid (TA) and gelatin (Gel) are both multi-functional molecules, the conjugation of TA onto Gel may lead to self-crosslinking, especially at high gelatin concentrations and high tannic acid feeding ratios, especially with porcine derived gelatin (PGel) that possesses high molecular weights and low water solubility. By adjusting gelatin concentrations and tannic acid feeding ratios, a family of tannic acid modified gelatin (Gel-TA, Scheme 1) was synthesized using low gelatin concentrations (up to 10 wt. %), 1–10% tannic acid (wt. % of gelatin), and various gelatin sources (fish, bovine, or porcine) in slightly basic water (pH 8.5). In this paper, both TA modified fish gelatin (derived from cold water fish skin) (FGel-TA) and TA modified gelatins from bovine skin (BGel-TA) were synthesized. NMR (Fig. 1A) and FTIR (Fig. 1B) spectra of FGel-TA polymers revealed characteristic peaks corresponding to the C-H protons (NMR) or C-H vibration (FTIR) on the benzene ring near the phenol –OH groups of TA (~6.8 ppm in NMR and ~1000 cm−1 in FTIR) that increased with higher TA feeding ratios ranging from 0, 1, 2, 5 to 10 wt%.
Fig. 1.
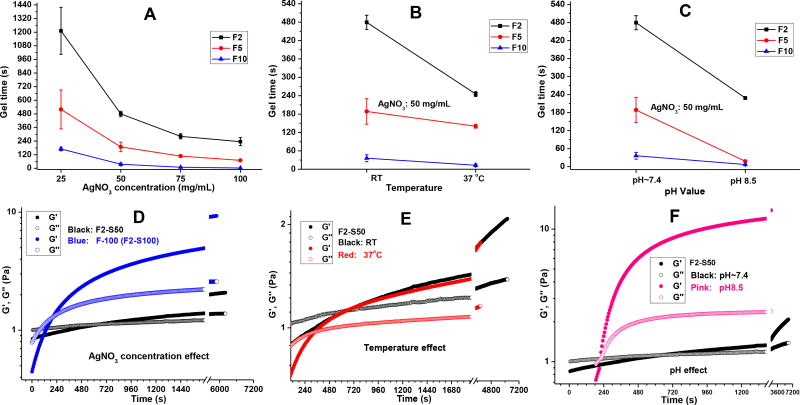
Following, we studied the gelation times of Gel-TA varying temperature, pH conditions, and crosslink initiator content. We chose silver nitrate (SN) as the crosslink initiator based on its compatibility with pyrogallol-functionalized polymers [41] and as an additional source of antimicrobial agent (Scheme 2). Overall, the gelation times ranged from 36s to 8 minutes, as tabulated in Table 2. Increasing SN content resulted in a decrease in gelation times among FGel-TA2 (F2), FGel-TA5 (F5), and FGel-TA10 (F10) (Fig. 2A), as supported by rheological studies (Fig. 2D). Increase in temperature from room to body temperature also decreased gelation times (Fig. 2B and 2E). Finally, increasing the pH conditions of gelatin solution from ~7.4 to 8.5 resulted in decreased gelation times for F2, F5, and F10, with F2 showing the most noticeable reduction in gelation time (Fig. 2C and 2F).
Fig. 2.
Mechanical characterization
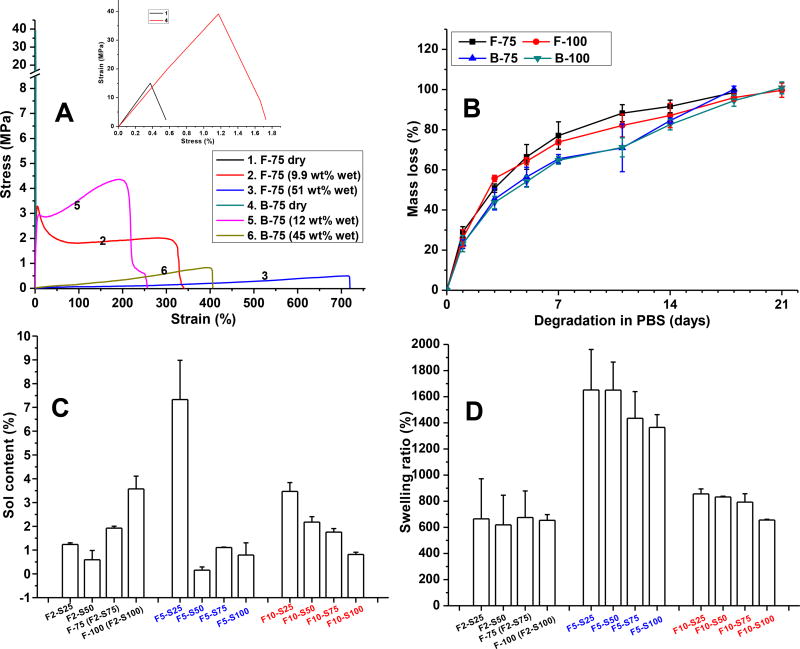
We then performed mechanical studies of dry and wet Gel-TA hydrogels crosslinked by silver nitrate (SN) (Gel-TA-SN) (Table 3 and Fig. 3A). Gelatins from different sources have different mechanical strengths; BGel itself possesses superior mechanical property compared to FGel. When Gel-TAs were crosslinked by 0.075 g/mL SN and at dry state, B-75 possessed much higher tensile strength (~40 MPa) than that of F-75 (~1.5 MPa). Both dry B-75 and F-75 were brittle with an elongation at break less than 2%. However, upon absorbing 9.9% (of dry polymer weight) of water, F-75 became soft and elastic, with a tensile strength of 2.97 ± 0.487 MPa and an elongation of 391 ± 19%. Increasing water absorption to 51% resulted in a significant improvement in elasticity with an elongation higher than 713 ± 54%, but with reduced tensile strength (0.5 ± 0.0 MPa) (Fig. 3A and Table 3). Likewise, B-75 exhibited improved elongation upon absorbing water (11.55 wt%), with even higher tensile strength (6.6 ± 0.8 MPa) than its fish-derived counterpart (Fig. 3A and Table 3). The above mechanical studies demonstrate the potential of tannin-derived gelatin hydrogels for applications ranging from topical use to tissue adhesives, as Gel-TA preserves sufficient mechanical strength upon water uptake while resembling the elasticity of soft tissue [42].
Table 3.
Mechanical properties of different gelatin-TA crosslinked by silver nitrate (SN) in dry and fully hydrated (swollen) states.
| Sample | Tensile strength (MPa) | Elongation at break (%) | Modulus (MPa) | |||
|---|---|---|---|---|---|---|
|
|
||||||
| Dry | Swollen | Dry | Swollen | Dry | Swollen | |
| F-75 | 33±8 | 3±0.5a | 1.1±0.1 | 391±19a | 3000±540 | 76±13a |
| F-75 | 0.50±0.0b | 713±54b | 0.12±0.0b | |||
| F-100 | 30±3 | - | 2.3±1.0 | - | 1600±500 | - |
| F5-S75 | 20±6 | 3.2±0.7a | 1.5±0.1 | 280±49a | 1400±360 | 102±44a |
| B-75 | 59±7 | 3.3±0.7a | 1.0±0.1 | 185±34a | 3700±730 | 48±10a |
| B-75 | 0.7±0.1b | 348±39b | 0.5±0.1b | |||
| B5-S75 | 6.6±0.8a | - | 111±11a | - | 173±14a | |
Film samples with water content around 10 wt% (for F2-S75 (F-75), 9.9 wt%, for F5-S75, 11.66 wt%, for B2-S75 (B-75), 12.0 wt%, for B5-S75, 11.55 wt%);
Film samples with water content around 50 wt% (for F-75, 51 wt%, for B-75, 45 wt%).
Fig. 3.
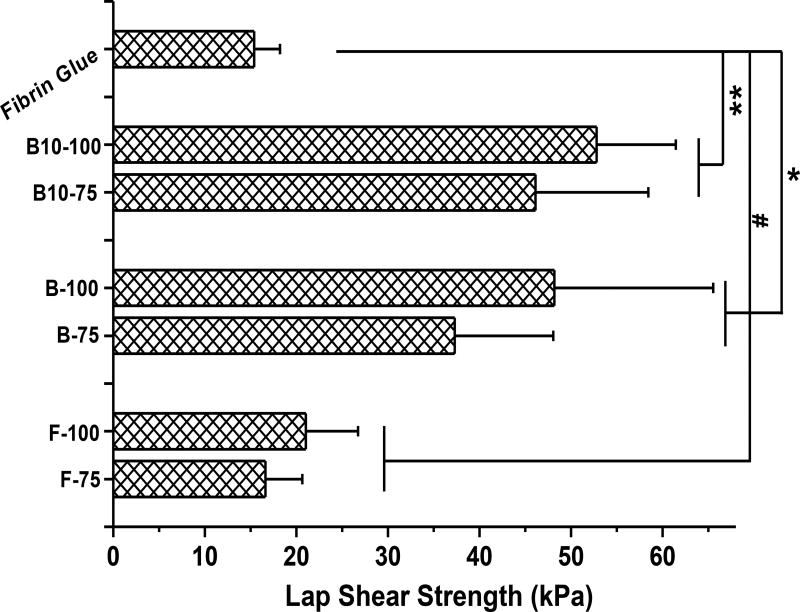
Subsequently, we sought to optimize the wet adhesion strength of our bioadhesive formulations. As shown in Fig. 4, the wet lap shear strengths of various Gel-TA-SN formulations ranged from 16.6 ± 4.0 kPa (for F-75) and 52.8 ± 8.7 kPa (for B-100). The lap shear strengths of FGel-TA-SN formulations to wet decellularized porcine small intestine submucosa (OASIS, HealthPoint Ltd. Fort Worth, TX) were close to that of the gold standard, fibrin glue [4, 5, 30], while the bovine-derived counterpart yielded even higher wet adhesion strengths, reflecting the stronger dry/wet mechanical properties above. Increasing of TA content also improved the adhesion strength of the crosslinked bioadhesives. The lap shear strengths of BGel-TA-SN formulations were between 2.5 to 3.7 times that of fibrin glue (15 ± 3 kPa), with the greatest adhesion strength from B-100 (around 55 kPa). Although the lap shear strength tests using decellularized small intestine submucosa tissues can give a direct comparison between the Gel-TA bioadhesives and the fibrin glue under the same test conditions, using real tissues for lap shear strength tests will be conducted in our future studies to better mimic in vivo tissue adhesion situations.
Fig. 4.
Assessment of biodegradability
All of the tannin-inspired gelatin adhesives showed favorable degradation, with full degradation within 3 weeks for crosslinked FGel-TA-SN and BGel-TA-SN (Fig. 3B). As with dopamine-derived polyphenol polymers in our previous studies [6], silver nitrate appears to impart faster degradation compared to other crosslink initiators such as sodium periodate. We hypothesize that the oxidized catechol groups of Gel-TAs chelate silver nanoparticles (reduced from silver nitrate), limiting the extent of intermolecular crosslinks and contributing to faster degradation.
Afterwards, we studied sol content and swelling behaviors of our crosslinked bioadhesives. The sol contents of FGel-TA crosslinked by different concentrations of silver nitrate (SN) were between 0–5%, with the exception of FGel-TA5-SN 0.025 g/mL (F5-S25) at 10% (Fig. 3C). As expected, higher crosslinking density led to lower sol content. The swelling ratios of the crosslinked FGel-TA-SN were in the range of 600% to 1600% (Fig. 3D). Compared to the FGel-TA2-SN formulations, FGel-TA5-SN formulations possessed higher swelling ratios regardless of SN content, likely because the ortho-quinone groups of oxidized tannic acid contributes to increased hydrophilicity. However, increasing TA content to 10% (e.g. FGel-TA10-SN) decreased swelling ratios, likely due to higher crosslinking densities.
Cytocompatibility of bioadhesive formulations
We next investigated the cytocompatibility of our Gel-TA-SN tissue adhesives, using FGel, FGel-TA, BGel, and BGel-TA, along with PEGDA as control (Fig. 5A). At 10 mg/mL concentrations, the hMSC viabilities of all Gel-TA polymers and gelatins were between 55 ± 7 and 98 ± 6%, comparable to that of control (at 57 ± 2%). Higher TA content led to lower cell viability. At 1.0 and 0.1 mg/mL concentrations, Gel-TA and gelatin performed even better than blank medium and PEGDA control in cell viability. The cytocompatibility of our bioadhesives was confirmed by Live/Dead assays. hMSCs seeded onto crosslinked Gel-TA-SN films showed excellent morphology, cell attachment and proliferation at 1, 3, and 7 day time points (Fig. 5D).
Fig. 5.
We also studied the cytotoxicity of leachable (sol) contents and degradation products of our crosslinked Gel-TA-SN hydrogels. At 1× dilution, Gel-TA-SN sol content resulted in roughly 35% cell viability, which improved to 90% at 10× dilution and ~100% at 100× dilution (Fig. 5B). The formation of silver nanoparticles likely contribute to the cytotoxicity of crosslinked Gel-TA-SN sol content. Likewise, Gel-TA-SN degradation products exhibited ~100% cell viability only upon 10× and 100× dilution (Fig. 5C).
Antimicrobial evaluation
In fruits and vegetables, tannins can act as natural antimicrobial agents to provide resistance against microorganisms [23]. The potential antibacterial activity of tannins against one of the most antibiotic drug-resistant “superbugs”, methicillin-resistant Staphylococcus aureus (MRSA) strain has been previously reported [28]. One possible antibacterial mechanisms of tannic acid was reported as the interactions of tannic acid with bacterial cell wall leading to the complexation with cell wall protein and membrane disruption metal ions [29]. Silver nanoparticles resulted from the redox reaction between oxidative of tannic acid with reductive silver nitrate (SN) could also serve as antimicrobial agents [5, 6, 35].
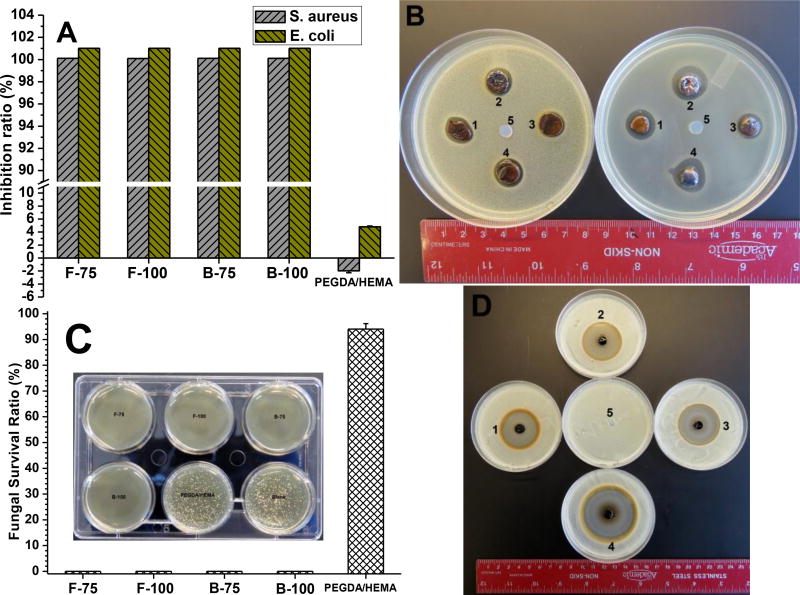
In chronic wounds such as diabetic foot ulcers, Streptococcus and S. aureus typically colonize and lead to clinical infections. Moreover, long-term chronic wounds tend to contain more anaerobes (such as Proteus and E. coli) than aerobes (such as Pseudomonas, Acinetobacter and Stenotrophomonas). Infected chronic wounds are also prone to fungal infections. Therefore, to study appropriate infection models for clinical treatment, we chose S. aureus and E. coli as representative Gram-positive and Gram-negative bacteria respectively, along with C. albicans as representative fungi, to evaluate the antimicrobial capabilities of our bioadhesives for the potential treatment of infected chronic wounds. Gel-TA-SN tissue adhesives were challenged with 1×106 CFU/mL of bacteria while PEGDA/HEMA served as control, and bacterial viabilities were assessed upon 24 hour incubation by the colony counting method (Fig. 6). The bacterial inhibition ratios of all Gel-TA-SN formulations (F75, F100, B75, and B100) were all close to 100% against both S. aureus and E. coli (Fig. 6A), indicating strong antibacterial performance of our bioadhesives. In contrast, the bacterial inhibition ratio of PEGDA/HEMA were close to 0 against both S. aureus and E. coli, indicating minimal inhibitory effects (Fig. 6A). We then performed the inhibition halo test as an additional assessment of microbial inhibition. The zones of bacterial inhibition around F-75, F-100, B-75, and B-100 hydrogels were all roughly 10 mm against S. aureus and between 12–20 mm against E. coli, while PEGDA/HEMA displayed no inhibition against either bacteria (Fig. 6B).
Fig. 6.
Similarly, fungal survival ratios upon direct exposure to Gel-TA-SN hydrogels were all 0% after 24 hour incubation of C. albicans, indicating strong fungal inhibition (Fig. 6C). Fungal survival ratio of PEGDA/HEMA as positive control was 98%. The zones of inhibition of F-75, F-100, B-75, and B-100 hydrogels against C. albicans were 36.6, 37.2, 38.9, and 51.6 mm respectively, while PEGDA/HEMA showed no zone of inhibition (Fig. 6D).
Although antimicrobial agents may be doped into a biomaterial to combat infections, these materials often suffer from burst release and poorly sustained action that can exacerbate surgical complications. Similar to the antimicrobial and antifungal iCMBAs that exhibited sustainable antimicrobial ability [6], the Gel-TA-SN system herein shows potential for short-term, highly effective antimicrobial activity from the fast-releasing silver nanoparticles, as well as for long-term, sustained action from tannic acid molecules incorporated into the gelatin network. Therefore, the dual integration of silver nanoparticles and tannic acid into a crosslinked polymeric network could be a powerful strategy for bioadhesive design with innate antimicrobial properties.
4. Conclusion
In conclusion, tannic acid (TA), as a representative of plant-derived polyphenol compound, was introduced into animal derived gelatin (gelatin from cold fish, FGel, or gelatin from bovine skin, BGel) through a facile one-step Michael addition reaction in aqueous solution at mild conditions to obtain gelatin-tannic acid (Gel-TA). Similar to mussel-inspired, dopamine containing polymers, Gel-TA can be crosslinked by oxidation, and the oxidized Gallic acid moieties on TA can also chemically bond to tissue by chemically reacting with nucleophilic groups such as –NH2 or –SH on tissue surface to serve as tissue adhesives. Upon crosslinking with silver nitrate (SN), the resulting tissue adhesives exhibited adjustable gel times, fast degradation, considerable wet tissue adhesion strengths, and favorable cytocompatibility. Gel-TA-SN tissue adhesives also demonstrated impressive antimicrobial properties. Compared to mussel-inspired bioadhesives that use expensive dopamine or DOPA as functional moieties, our polyphenol based Gel-TA tissue adhesives utilize tannic acid as an abundant and low-cost raw material, and eliminate the concerns of potential neurological effect brought by dopamine. Therefore, the studies herein suggest potential applications of the tannin-inspired gelatin bioadhesives toward wound closure, tissue sealant, hemostasis, antimicrobial, and cell/drug delivery. Further research will be necessary to evaluate these materials in various animal models toward clinical translation.
Statement of Significance.
This manuscript describes the development of a new family of tannin-inspired antimicrobial bioadhesives derived from a facile, one-step Michael addition of tannic acid and gelatin under oxidizing conditions and crosslinked by silver nitrate. Our strategy is new and can be easily extended to other polymer systems, low-cost and readily scalable, and eliminate the concerns of potential neurological effect brought by mussel-inspired strategy due to the inclusion of dopamine.
The tannin-inspired gelatin bioadhesives hold great promise for a number of applications in wound closure, tissue sealant, hemostasis, antimicrobial and cell/drug delivery, and would be interested to the readers from biomaterials, tissue engineering, and drug delivery area.
Acknowledgments
This work was supported in part by a National Cancer Institute (NCI) Award CA182670 and a National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) award AR071316. The authors would like to thank Dr. Yilan Ye, Prof. Ralph Colby from The Department of Material Science and Engineering, The Pennsylvania State University for their help on rheology tests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Annabia N, Tamayol A, Shin SR, Ghaemmaghamid AM, Peppase NA, Khademhosseini A. Surgical materials: Current challenges and nano-enabled solutions. Nano. Today. 2014;9:574–589. doi: 10.1016/j.nantod.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kazemzadeh-Narbat M, Annabi N, Khademhosseini A. Surgical sealants and high strength adhesives. Mater. Today. 2015;18:176–177. [Google Scholar]
- 3.Mehdizadeh M, Yang J. Design strategies and applications of tissue bioadhesives. Macromol. Biosci. 2013;13:271–288. doi: 10.1002/mabi.201200332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehdizadeh M, Weng H, Gyawali D, Tang L, Yang J. Injectable citrate-based mussel-inspired tissue bioadhesives with high wet strength for sutureless wound closure. Biomaterials. 2012;33:7972–7983. doi: 10.1016/j.biomaterials.2012.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo J, Kim GB, Shan D, Kim JP, Hu J, Wang W, Hamad FG, Qian G, Rizk EB, Yang J. Click chemistry improved wet adhesion strength of mussel-inspired citrate-based antimicrobial bioadhesives. Biomaterials. 2017;112:275–286. doi: 10.1016/j.biomaterials.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo J, Wang W, Hu J, Xie D, Gerhard E, Nisic M, Shan D, Qian G, Zheng S, Yang J. Synthesis and characterization of anti-bacterial and anti-fungal citrate-based mussel-inspired bioadhesives. Biomaterials. 2016;85:204–217. doi: 10.1016/j.biomaterials.2016.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie D, Guo J, Mehdizadeh MR, Tran RT, Chen R, Sun D, Qian G, Jin D, Bai X, Yang J. Development of injectable citrate-based bioadhesive bone implants. J. Mater. Chem. B. 2015;3:387–398. doi: 10.1039/C4TB01498G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrett DG, Bushnell GG, Messersmith PB. Mechanically robust, negative-swelling, mussel-inspired tissue adhesives. Adv. Healthcare Mater. 2013;2:745–755. doi: 10.1002/adhm.201200316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerdá DC, Ballester AM, Aliena-Valero A, Carabén-Redaño A, Lloris JM. Use of cyanoacrylate adhesives in general surgery. Surg. Today. 2015;45:939–956. doi: 10.1007/s00595-014-1056-4. [DOI] [PubMed] [Google Scholar]
- 10.Beckman EJ, Buckley M, Agarwal S, Zhang J. Medical adhesive and methods of tissue adhesion. 2007 US7264823B2. [Google Scholar]
- 11.Gill SK, Roohpour N, Topham PD, Tighe BJ. Tunable denture adhesives using biomimetic principles for enhanced tissue adhesion in moist environments. Acta. Biomater. 2017;63:326–335. doi: 10.1016/j.actbio.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Guo J, Xie Z, Tran RT, Xie D, Jin D, Bai X, Yang J. Click chemistry plays a dual role in biodegradable polymer design. Adv. Mater. 2014;26:1906–1911. doi: 10.1002/adma.201305162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu J, Guo J, Xie Z, Shan D, Gerhard E, Qian G, Yang J. Fluorescence imaging enabled poly(lactide-co-glycolide) Acta. Biomater. 2016;29:307–319. doi: 10.1016/j.actbio.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shan D, Zhang C, Kalaba S, Mehta N, Kim GB, Liu Z, Yang J. Flexible biodegradable citrate-based polymeric step-index optical fiber. Biomaterials. 2017;143:142–148. doi: 10.1016/j.biomaterials.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan C, Fu J, Zhu W, Wang DA. A mussel-inspired double-crosslinked tissue adhesive intended for internal medical use. Acta. Biomater. 2016;33:51–63. doi: 10.1016/j.actbio.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Schultz W. Multiple dopamine functions at different time courses. Annu. Rev. Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- 17.Sileika TS, Barrett DG, Zhang R, Lau KHA, Messersmith PB. Colorless multifunctional coatings inspired by polyphenols found in tea, chocolate, and wine. Angew. Chem. Int. Ed. 2013;52:10766–10770. doi: 10.1002/anie.201304922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Messersmith PB, Sileika TS, Zhang R, Barrett DG. Phenolic coatings and methods of making and using same. 2014 US20140206630. [Google Scholar]
- 19.Zhang X, Do MD, Casey P, Sulistio A, Qiao GG, Lundin L, Lillford P, Kosaraju S. Chemical modification of gelatin by a natural phenolic cross-linker, tannic acid. J. Agric. Food. Chem. 2010;58:6809–6815. doi: 10.1021/jf1004226. [DOI] [PubMed] [Google Scholar]
- 20.Bedran-Russo AK, Yoo KJ, Ema KC, Pashley DH. Mechanical properties of tannic-acid-treated dentin matrix. J. Dent. Res. 2009;88:807–811. doi: 10.1177/0022034509342556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strauss G, Gibson SM. Plant phenolics as cross-linkers of gelatin gels and gelatin-based coacervates for use as food ingredients. Food Hydrocolloid. 2004;18:81–89. [Google Scholar]
- 22.Scalbert A. Antimicrobial properties of tannins. Phytochemistry. 1991;30:3875–3883. [Google Scholar]
- 23.Cowan MM. Plant products as antimicrobial agents. Clin. Microbiol. Rew. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung KT, Lu Z, Chou MW. Mechanism of inhibition of tannic acid and related compounds on the growth of intestinal bacteria. Food Chem. Toxicol. 1998;36:1053–1060. doi: 10.1016/s0278-6915(98)00086-6. [DOI] [PubMed] [Google Scholar]
- 25.Simon SA, Disalvo EA, Gawrisch K, Borovyagin V, Toone E, Schiffman SS, Needham D, McIntosh TJ. Increased adhesion between neutral lipid bilayers: interbilayer bridges formed by tannic acid. Biophys. J. 1994;66:1943–1958. doi: 10.1016/S0006-3495(94)80988-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan H, Wang L, Feng X, Bu Y, Wu D, Jin Z. Supramolecular hydrogel formation based on tannic acid. Macromolecules. 2017;50:666–676. [Google Scholar]
- 27.Oh HI, Hoff JE, Armstrong GS, Haff LA. Hydrophobic interaction in tannin-protein complexes. J. Agric. Food Chem. 1980;28:394–398. [Google Scholar]
- 28.Kyaw BW, Arora S, Lim CS. Bactericidal antibiotic-phytochemical combinations against methicillin resistant Staphylococcus aureus. Braz. J. Microbial. 2012;43:938–945. doi: 10.1590/S1517-838220120003000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kyaw BM, Lim CS, Zhou W. Tannic acid as phytochemical potentiator for antibiotic resistance adaptation. APCBEE Procedia. 2013;7:175–181. [Google Scholar]
- 30.Assmann A, Vegh A, Ghasemi-Rad M, Bagherifard S, Cheng G, Sani ES, Ruiz-Esparza GU, Noshadi I, Lassaletta AD, Gangadharan S, Tamayol A, Khademhosseini A, Annabi N. A highly adhesive and naturally derived sealant. Biomaterials. 2017;140:115–127. doi: 10.1016/j.biomaterials.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanders L, Stone R, Webb CK, Mefford OT, Nagatomi J. Mechanical Characterization of a Bi-functional Tetronic Hydrogel Adhesive for Soft Tissues. J. Biomed. Mater. Res. A. 2015;103:861–868. doi: 10.1002/jbm.a.35310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su LC, Xie Z, Zhang Y, Nguyen KT, Yang J. Study on the antimicrobial properties of citrate-based biodegradable polymers. Front. Bioeng. Biotechnol. 2014;2:23. doi: 10.3389/fbioe.2014.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou Y, Zhao Y, Wang L, Xu L, Zhai Z, Wei S. Radiation synthesis and characterization of nanosilver/gelatin/carboxymethyl chitosan hydrogel. Radiat. Phys. Chem. 2012;81:553–560. [Google Scholar]
- 34.Pundir PK, Jain P. Evaluation of five chemical food preservatives for their antibacterial activity against bacterial isolates from bakery products and mango pickles. J. Chem. Pharm. Res. 2011;3:24–31. [Google Scholar]
- 35.Hudson SP, Langer R, Fink GR, Kohane DS. Injectable in situ cross-linking hydrogels for local antifungal therapy. Biomaterials. 2010;31:1444–1452. doi: 10.1016/j.biomaterials.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakai S, Hirose K, Taguchi K, Ogushi Y, Kawakami K. An injectable, in situ enzymatically gellable, gelatin derivative for drug delivery and tissue engineering. Biomaterials. 2009;30:3371–3377. doi: 10.1016/j.biomaterials.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 37.Fu J, Quek KY, Chuah YL, Lim CS, Fan C, Wang D. The effects of gelatin–dopamine coating on polydimethylsiloxane substrates on pluripotency maintenance and myocardial differentiation of cultured mouse embryonic stem cells. J. Mater. Chem. B. 2016;4:7961–7973. doi: 10.1039/c6tb02631a. [DOI] [PubMed] [Google Scholar]
- 38.Thiruselvi T, Thirupathi KRS, Aravindhan R, Shanuja SK, Gnanamani A. Handling and managing bleeding wounds using tissue adhesive hydrogel: A comparative assessment on two different hydrogels. RSC Adv. 2016;6:19973–19981. doi: 10.1039/c9ra90054c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cammarata CR, Hughes ME, Ofner CM., III Carbodiimide induced cross-linking, ligand addition, and degradation in gelatin. Mol. Pharmaceutics. 2015;12:783–793. doi: 10.1021/mp5006118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koshy ST, Ferrante TC, Lewin SA, Mooney DJ. Injectable, porous, and cell-responsive gelatin cryogels. Biomaterials. 2014;35:2477–2487. doi: 10.1016/j.biomaterials.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fullenkamp DE, Rivera JG, Gong YK, Lau KHA, He L, Varshney R, Messersmith PB. Mussel-inspired silver-releasing antibacterial hydrogels. Biomaterials. 2012;33:3783–3791. doi: 10.1016/j.biomaterials.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen QZ, Bismarck A, Hansen U, Junaid S, Tran MQ, Harding SE, Ali NN, Boccaccini AR. Characterisation of a soft elastomer poly(glycerol sebacate) designed to match the mechanical properties of myocardial tissue. Biomaterials. 2008;29:47–57. doi: 10.1016/j.biomaterials.2007.09.010. [DOI] [PubMed] [Google Scholar]