Figure 1b.
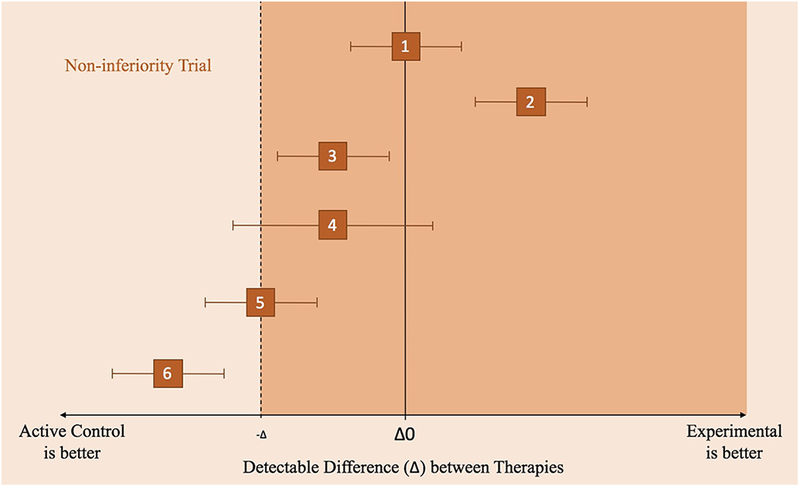
Non-inferiority Clinical Trial Principles illustrated by different scenarios (1–6), where the effects of an active control and experimental treatment are within the confidence intervals (along the x-axis).
1) Experimental non-inferior to control but not significantly different from active control.
2) Experimental is non-inferior to control but is significantly different from control.
3) Experimental is somewhat inferior to control. Experimental is not inferior because the CI does not include the non-inferior margin or the line of Δ0.
4) Inconclusive because the CI includes values on both sides of the non-inferiority margin and the line of no treatment difference (Δ0).
5) Experimental is inferior to control because it includes the non-inferiority margin.
6) Experimental is inferior to control and is more certain of its inferiority as compared to scenario (5) because it does not include the non-inferiority margin.
