Table 1.
Substrate overview with yields; product denominations: a, alcohol; e, cyclic ether; i, internal alkene; t, terminal alkene; all reactions run in filtered CDCl3.
| Entry | Substrate | Products | Background | |
|---|---|---|---|---|
| 1 | 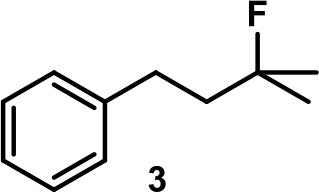 |
3i 69%a (16 h) 3t 13%a | 0b 0c | |
| 2 | 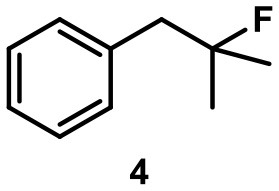 |
4i 45%a (16 h) 4a 35%a 4t 16%a | 0b 0c | |
| 3 | 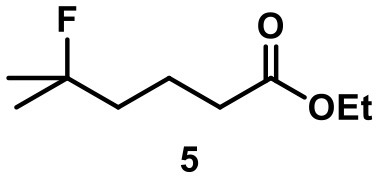 |
5I 60%a (20 h) 5i 29%a 5t 3%a | 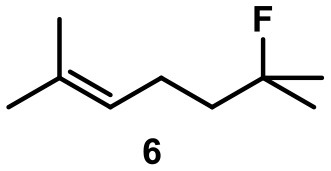 |
0b 0c |
| 4 | 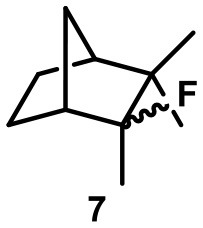 |
6e 81%a (21 h) 6i 11%a | 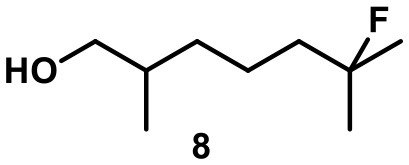 |
0b 0c |
| 5 | 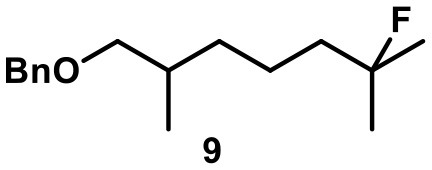 |
7t 98%a (2 h) | 7t 98%b (20 h) 0c | |
| 6 | 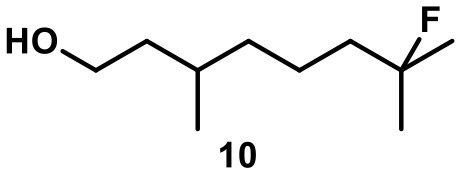 |
8e 72%a (4 h) | 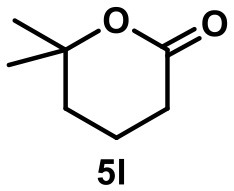 |
8e 71%b (7d) 0c |
| 7 | 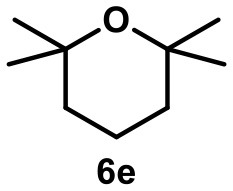 |
9i 65%a(4 h) 9t 6%a | 9i 5%b(7d) 0c | |
| 8 | 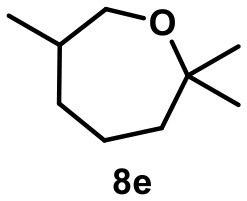 |
10i 60%a (4 h) 10a 6%a 10t 16%a | 10i 52%b (7d) 10a 6%b 10t 13%a 0c |
All reactions run in CDCl3 (filtered over basic Al2O3 prior to use) at 40°C; adetermined via 1H NMR baddition of TBAB (1.5 eq. relative to capsule I), reaction run for 7 d at 40°C c addition of HOAc (10 mol%), no hexamer present, 7d, 40°C.
