Table 1.
Polymerization of L-Lactide and ε-caprolactone using complexes 1–2, 4 at 50°Ca.
| Entry | Monomer | Solvent | Cat. | {[M]0:[Zn]0}:[BnOH] | Time (min) | Mn (obsd)b | Mn (calcd)c | Conv.(%)d | Yield (%)e | Mw/ |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 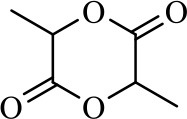 |
Toluene | 1 | 50:1 | 15 | 10,600 (6,200) | 6,900 | 94 | 79 | 1.16 |
| 2 | Toluene | 2 | 50:1 | 15 | 11,800 (6,800) | 6,900 | 94 | 75 | 1.07 | |
| 3 | Toluene | 4 | 50:1 | 120 | – | – | 15 | – | – | |
| 4 | Toluene | 4 | 50:1 | 1,440 | 21,000 (12,200) | 7,200 | 98 | 82 | 1.38 | |
| 5 | Toluene | 1 | 200:1 | 45 | 40,300 (23,400) | 28,400 | 98 | 86 | 1.35 | |
| 6 | Toluene | 1 | 300:1 | 50 | 27,700 (16,100) | 42,100 | 97 | 88 | 2.20 | |
| 7 | Toluene | 2 | 100:1 | 15 | 28,000 (16,200) | 13,500 | 93 | 82 | 1.05 | |
| 8 | Toluene | 2 | 150:1 | 30 | 34,100 (19,800) | 18,900 | 87 | 73 | 1.09 | |
| 9 | Toluene | 2 | 200:1 | 45 | 46,200 (26,800) | 27,200 | 94 | 84 | 1.21 | |
| 10 | Toluene | 2 | 300:1 | 50 | 38,200 (22,200) | 41,600 | 96 | 83 | 1.35 | |
| 11f | 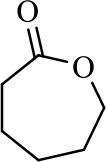 |
THF | 2 | 50:1 | 120 | 6,600 (3,700) | 5,200 | 90 | 78 | 1.21 |
| 12f | DCM | 2 | 50:1 | 120 | – | – | Trace | – | – | |
| 13f | Toluene | 2 | 50:1 | 120 | 15,100 (8,500) | 5,800 | 99 | 80 | 1.22 | |
| 14f | THF | 1 | 50:1 | 120 | – | – | 62 | – | – | |
| 15f | DCM | 1 | 50:1 | 120 | – | – | 13 | – | – | |
| 16f | Toluene | 1 | 50:1 | 120 | 14,100 (7,900) | 5,500 | 95 | 80 | 1.35 | |
| 17f | THF | 4 | 50:1 | 120 | – | – | 60 | – | – | |
| 18f | DCM | 4 | 50:1 | 120 | – | – | 27 | – | – | |
| 19f | Toluene | 4 | 50:1 | 120 | 10,300 (5,800) | 4,400 | 75 | – | 1.12 | |
| 20 | Toluene | 1 | 50:1 | 8 | 11,100 (6,200) | 4,900 | 84 | 72 | 1.14 | |
| 21 | Toluene | 2 | 50:1 | 8 | 11,200 (6,300) | 5,600 | 97 | 83 | 1.08 | |
| 22 | Toluene | 4 | 50:1 | 60 | 4,900 (2,700) | 5,800 | 99 | 78 | 1.16 | |
| 23 | Toluene | 2 | 100:1 | 15 | 21,800 (12,200) | 11,000 | 95 | 82 | 1.11 | |
| 24 | Toluene | 2 | 150:1 | 20 | 32,300 (18,100) | 16,900 | 98 | 87 | 1.27 |
For L-Lactide: 10 mL solvent, [Zn]0 = 0.005M, [BnOH] = 0.005M; for ε-caprolactone: 15 mL solvent. [Zn]0 = 8.33mM; [BnOH] = 8.33mM.
Obtained from GPC analysis and calibrated by polystyrene standard. Values in parentheses are the values obtained from GPC times 0.58 for PLA; 0.56 for PCL.
Calculated from [Mw(monomer) × [M]0/[Zn]0 × conversion /([BnOH](eq)] + M(BnOH)).
Obtained from 1H NMR analysis.
Isolated yield.
T = 26°C.
