Fig. 1.
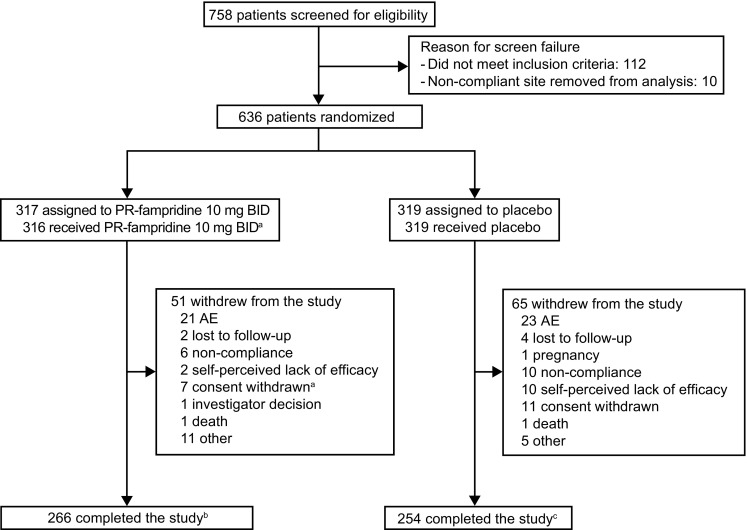
Participant disposition. AE adverse event, BID twice daily, PR prolonged-release. a1 patient randomized to PR-fampridine withdrew from the study before dosing (reason: consent withdrawn). bPatients who completed the 24-week double-blind treatment period and 2-week off-treatment follow-up visit calculated as follows: 317 − 51 = 266. A total of 271 participants completed the 24-week treatment period (Weeks 0–24). cPatients who completed the 24-week double-blind treatment period and 2-week off-treatment follow-up visit calculated as follows: 319 − 65 = 254. A total of 258 participants completed the 24-week treatment period (Weeks 0–24)
